Glucose, one of a group of carbohydrates known as simple sugars (monosaccharides).
What is Glucose
The group of compounds known as carbohydrates received their general name because of early observations that they often have the formula Cx(H2O)y - that is, they appear to be hydrates of carbon. One of the carbohydrates is known as Monosaccharide (The monosaccharides are polyhydroxy aldehydes or polyhydroxy ketones which cannot be decomposed by hydrolysis to give simpler carbohydrates) Glucose is one of the monosaccharides.
Also Check: Baking Soda | Epsom salt
Structure of Glucose
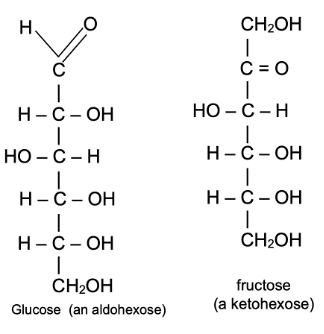
Reactions of Glucose
- Mild Oxidation: Mild oxidation of aldoses gives aldonic acid. Br2 water or alkaline solution of iodine oxidises only the aldehydic group to give aldonic acids.
- Strong Oxidation: Strong oxidation of aldoses oxidise both -CHO group and terminal -CH2OH group into -COOH to give aldaric acid.
- Reduction of Sugar: Sugar can be reduced into corresponding alcohols by variety of reducing agents like high pressure catalytic hydrogenation (Ni/H2), NaBH4, (iii) Na/Hg (iv) electrolytic reduction in acidic medium.
- Reaction of Aldose and Ketose with Phenyl Hydrazine: Aldose and ketose both react with phenyl hydrazine (excess) to form osazones, which contain two phenylhydrazone group and also give aniline and ammonia.
Relation between starch and Glucose
A polymer of glucose, a major fuel store in plants, but is absent from animals where the equivalent is glycogen, can easily be converted back to glucose for use in respiration. In germinating seeds the glucose may also be used to make cellulose and other materials needed for growth.
Starch has two components, amylose (20-30% of starch) and amylopectin (70-80% of starch). In wrinkle seeded pea, amylose fraction is upto 98%, while waxy starch of maize may be almost entirely made of amylopectin.Amylose has a straight chain structure consisting of several thousand glucose residues joined by 1, 4 bonds. These bonds cause the chain to coil helically into a more compact shape.
Amylopectin is also compact as it has many branches, formed by 1, 6 glycosidic bonds. It has up to twice as many glucose residues as amylose. A suspension of amylose in water gives a blue-black colour with iodine-(potassium iodide solution), whereas a suspension of amylopectin gives a red-violet colour. This forms the basis of the test for starch.