About Chapter 3: Chemical Reactions and Equations Notes – Class 10 Chemistry
Chemical reactions form the core of Chemistry, as they explain how substances interact to form new products. This chapter introduces chemical equations, types of reactions, and their applications.
A chemical equation represents the reactants and products with their symbols. For example, H₂ + O₂ → H₂O. Balancing chemical equations ensures that the law of conservation of mass is followed. Students must learn stepwise balancing methods to score well in exams. One must go through the NCERT textbook for class 10 and solve the questions with the help of the NCERT solutions for class 10 science.
Types of chemical reactions include:
-
Combination reactions: two or more substances combine to form a single product.
-
Decomposition reactions: a compound breaks down into simpler products using heat, electricity, or light.
-
Displacement reactions: a more reactive element displaces a less reactive one from its compound.
-
Double displacement reactions: two compounds exchange ions to form new products, often resulting in precipitates.
-
Redox reactions: involving both oxidation (loss of electrons) and reduction (gain of electrons).
Examples include the rusting of iron (a slow redox reaction) and the photosynthesis process in plants (a complex combination reaction). The Class 10 chapter also highlights the effects of oxidation and reduction in everyday life. Corrosion, where metals react with oxygen and moisture, weakens iron and steel structures. Rancidity, caused by the oxidation of oils and fats, spoils food, which is why antioxidants and proper storage methods are used to prevent it. Students also learn the importance of chemical equations in industries. For example, neutralisation reactions are used in pharmaceuticals, displacement reactions in metallurgy, and decomposition reactions in the cement industry. This chapter sharpens the ability to classify and predict reactions, balance equations accurately, and apply theoretical concepts to real-life examples, making it crucial for board exam preparation.
Understanding Chemical Reactions: Types, Equations, and Practical Applications
Chemical reactions form the foundation of chemistry and are essential processes that transform substances into new materials with different properties. Whether it's the rusting of iron, the digestion of food in our bodies, or the burning of fuel, chemical reactions are occurring constantly around us. This comprehensive guide explores the fundamental concepts of chemical reactions, how to represent them through balanced equations, and their significant impact on everyday life.
What is a Chemical Reaction?
A chemical reaction is a process that transforms one or more substances (reactants) into new substances (products) with different properties. During these reactions, chemical bonds break and reform, resulting in substances with new names and chemical formulas.
Key Characteristics of Chemical Reactions
Chemical reactions exhibit several distinctive features including evolution of gas, change of color, formation of precipitate, energy changes (heat, light, or electricity), and change of state. For example, when zinc metal reacts with dilute sulfuric acid, hydrogen gas evolves with effervescence, and when copper carbonate is heated, it changes from green to black as it decomposes.
Chemical Equations: Representing Reactions Symbolically
A chemical equation is a symbolic representation of an actual chemical change using symbols and formulas of reactants and products. These equations provide a concise way to communicate complex chemical transformations.
Balanced vs. Unbalanced Chemical Equations
A balanced chemical equation has equal numbers of atoms of each element on both the reactant and product sides, satisfying the law of conservation of mass. The law of conservation of mass states that the total mass of elements in products must equal the total mass of elements in reactants.
An unbalanced (skeletal) equation has unequal numbers of atoms of different elements on the reactant and product sides. For instance, the equation Al + O₂ → Al₂O₃ is unbalanced because there is 1 aluminum atom on the left but 2 on the right, and 2 oxygen atoms on the left but 3 on the right.
How to Balance Chemical Equations
Balancing involves adjusting coefficients before symbols or formulas so that the total number of atoms of each element becomes equal on both sides. The process follows these steps:
- Write the word equation showing reactants and products
- Convert to symbol equation using correct formulas
- Count atoms of each element on both sides
- Adjust coefficients (never change formulas) to balance atoms
- Add state symbols and conditions to make the equation informative
State symbols include (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous solution. Arrows pointing upward (↑) indicate gas evolution, while downward arrows (↓) indicate precipitate formation.
Types of Chemical Reactions
Chemical reactions are classified into several categories based on how reactants transform into products. Understanding these types helps predict reaction outcomes and balance equations effectively.
1. Combination Reactions
Combination reactions involve two or more substances combining to form a single substance. These are also called synthesis reactions when elements unite to form compounds.
Examples:
- 2H₂(g) + O₂(g) → 2H₂O(l) - Formation of water
- 2Mg(s) + O₂(g) → 2MgO(s) - Burning of magnesium
- CaO(s) + H₂O(l) → Ca(OH)₂(aq) - Quick lime reacting with water (exothermic)
2. Decomposition Reactions
Decomposition reactions involve a single substance splitting into two or more simpler substances, typically requiring energy in the form of heat (thermolysis), electricity (electrolysis), or light (photolysis).
Thermal Decomposition
When ferrous sulfate crystals (green) are heated, they first lose water to form anhydrous ferrous sulfate (white), then decompose further into ferric oxide (brown) with the smell of burning sulfur.
Key examples:
- 2FeSO₄(s) → Fe₂O₃(s) + SO₂(g) + SO₃(g)
- 2KClO₃(s) → 2KCl(s) + 3O₂(g) (with MnO₂ catalyst)
- 2Pb(NO₃)₂(s) → 2PbO(s) + 4NO₂(g) + O₂(g) - Lead nitrate turning from colorless to yellow
Electrolytic Decomposition
Electrolysis uses electric current to decompose compounds, such as water splitting into hydrogen and oxygen gases. The volume of hydrogen collected is double that of oxygen because two hydrogen atoms and one oxygen atom make up one water molecule.
Photo-decomposition
Photolysis reactions occur when light energy breaks down compounds. Silver chloride decomposes in sunlight, turning from white to gray as silver metal forms. This principle is why hydrogen peroxide is stored in colored bottles.
3. Displacement Reactions
Displacement reactions involve a more reactive element displacing a less reactive element from its compound. The activity series arranges metals in decreasing order of reactivity, with potassium being most reactive and platinum least reactive.
Important principles:
- Metals above hydrogen in the activity series can displace hydrogen from acids or water
- Any metal can displace metals lying below it in the series from their solutions
- For non-metals like halogens: F > Cl > Br > I in reactivity
Classic example: When iron nails are placed in copper sulfate solution (blue), the solution fades to light green, and a reddish-brown deposit of copper forms on the iron. This demonstrates iron displacing copper: Fe(s) + CuSO₄(aq) → FeSO₄(aq) + Cu(s)
4. Double Displacement Reactions
Double displacement reactions involve two ionic compounds exchanging ions to form new compounds, typically occurring in solution.
Examples:
- BaCl₂(aq) + Na₂SO₄(aq) → BaSO₄(s) + 2NaCl(aq) - White precipitate of barium sulfate
- Pb(NO₃)₂(aq) + 2KI(aq) → PbI₂(s) + 2KNO₃(aq) - Yellow precipitate of lead iodide
These are also called precipitation reactions because they produce insoluble precipitates.
Oxidation and Reduction: Redox Reactions
Oxidation and reduction are fundamental concepts that describe electron transfer and changes in chemical composition during reactions.
Understanding Oxidation
Oxidation is a chemical reaction in which a substance gains oxygen, loses hydrogen, gains electronegative elements, or loses electropositive elements.
Three definitions of oxidation:
- In terms of oxygen: Addition of oxygen or electronegative elements to a substance
- Example: 2Mg + O₂ → 2MgO (oxygen added to magnesium)
- In terms of hydrogen: Removal of hydrogen or electropositive elements from a substance
- Example: H₂S + Cl₂ → 2HCl + S (hydrogen removed from hydrogen sulfide)
- Electronic concept: Loss of electrons, resulting in increased positive charge or decreased negative charge
- Example: Na → Na⁺ + e⁻ (sodium loses an electron)
Understanding Reduction
Reduction is a chemical reaction involving gain of hydrogen or electropositive radicals, or loss of oxygen or electronegative radicals.
Three definitions of reduction:
- In terms of oxygen: Removal of oxygen or electronegative elements
- Example: CuO + H₂ → Cu + H₂O (oxygen removed from copper oxide)
- In terms of hydrogen: Addition of hydrogen or electropositive elements
- Example: Cl₂ + H₂ → 2HCl (hydrogen added to chlorine)
- Electronic concept: Gain of electrons, resulting in increased negative charge or decreased positive charge
- Example: Cl₂ + 2e⁻ → 2Cl⁻ (chlorine gains electrons)
Redox Reactions
Redox reactions are those in which oxidation and reduction occur simultaneously. Neither process can occur without the other.
Classic example: When hydrogen gas is passed over heated copper oxide, the black copper oxide turns brown as it's reduced to copper metal, while hydrogen is oxidized to water.
CuO + H₂ → Cu + H₂O
In this reaction:
- CuO is reduced (loses oxygen) - CuO acts as the oxidizing agent
- H₂ is oxidized (gains oxygen) - H₂ acts as the reducing agent
Oxidation Reactions in Everyday Life
Chemical reactions, particularly oxidation processes, play crucial roles in our daily experiences, both beneficial and harmful.
Combustion Reactions
Combustion reactions involve substances burning or oxidizing in the presence of air or oxygen, releasing heat energy. All combustion reactions are exothermic and provide energy for various applications.
Examples:
- Methane combustion: CH₄ + 2O₂ → CO₂ + 2H₂O + heat
- Glucose combustion in the body: C₆H₁₂O₆ + 6O₂ → 6CO₂ + 6H₂O + energy
Respiration: Biochemical Oxidation
Respiration is the most important biochemical reaction that releases energy in cells through the combustion of glucose with oxygen. This exothermic process provides warmth and energy for cellular functions, keeping our hearts and muscles working.
Corrosion: Unwanted Oxidation
Corrosion is the slow eating away of metal surfaces when exposed to moist air for extended periods. This process causes significant economic damage to buildings, railways, automobiles, and infrastructure.
Rusting of Iron
Rusting occurs when iron reacts with oxygen and water vapor in moist air, forming hydrated ferric oxide (Fe₂O₃·xH₂O), a brown flaky substance.
Reaction: 4Fe(s) + 3O₂(g) + 2xH₂O(l) → 2Fe₂O₃·xH₂O(s)
Rusting is problematic because the rust doesn't adhere to the surface; it falls off, exposing fresh iron to continued corrosion.
Prevention methods:
- Painting iron objects like gates and car bodies
- Greasing and oiling machine parts
- Galvanization - coating iron with zinc, which is more corrosion-resistant
Corrosion of Other Metals
Copper develops a green coating of basic copper carbonate (CuCO₃·Cu(OH)₂) when exposed to moist air containing oxygen, carbon dioxide, and water vapor. Silver tarnishes with a black coating, another example of metal corrosion.
Essential Chemical Reaction Formulas
| Reaction Type | General Formula | Example | Description |
|---|---|---|---|
| Combination | A + B → AB | 2Mg + O₂ → 2MgO | Two or more reactants form single product |
| Decomposition (Thermal) | AB → A + B (+ heat) | 2FeSO₄ → Fe₂O₃ + SO₂ + SO₃ | Single reactant breaks into multiple products with heat |
| Decomposition (Electrolytic) | AB → A + B (+ electricity) | 2H₂O → 2H₂ + O₂ | Electric current splits compound |
| Decomposition (Photo) | AB → A + B (+ light) | 2AgCl → 2Ag + Cl₂ | Light energy breaks chemical bonds |
| Displacement | A + BC → AC + B | Fe + CuSO₄ → FeSO₄ + Cu | More reactive element replaces less reactive |
| Double Displacement | AB + CD → AD + CB | BaCl₂ + Na₂SO₄ → BaSO₄ + 2NaCl | Exchange of ions between two compounds |
| Oxidation | A + O₂ → AO | 2Cu + O₂ → 2CuO | Addition of oxygen or loss of hydrogen |
| Reduction | AO + H₂ → A + H₂O | CuO + H₂ → Cu + H₂O | Removal of oxygen or gain of hydrogen |
| Redox | Oxidation + Reduction | CuO + H₂ → Cu + H₂O | Simultaneous oxidation and reduction |
| Combustion | Fuel + O₂ → CO₂ + H₂O + Energy | CH₄ + 2O₂ → CO₂ + 2H₂O | Burning with oxygen releasing energy |
Law of Conservation of Mass
All chemical equations must be balanced to satisfy the law of conservation of mass, which states that the total mass of products equals the total mass of reactants. For example, when calcium carbonate (100g) decomposes, it produces calcium oxide (56g) and carbon dioxide (44g), totaling 100g demonstrating that matter is never destroyed.
Understanding chemical reactions is essential for comprehending the natural world and technological processes. From the food we digest to the materials that corrode around us, chemical reactions shape our daily experiences. By mastering concepts like balancing equations, identifying reaction types, and understanding oxidation-reduction processes, students gain powerful tools for predicting and controlling chemical changes. Whether preventing rust, harnessing combustion energy, or simply understanding how our bodies extract energy from food, the principles of chemical reactions provide the foundation for chemistry and numerous practical applications in science, industry, and everyday life.
PHYSICAL AND CHEMICAL CHANGES
CHANGES IN MATTER:
Changes in matter can be studied in two major ways. The two types of changes are as follows:
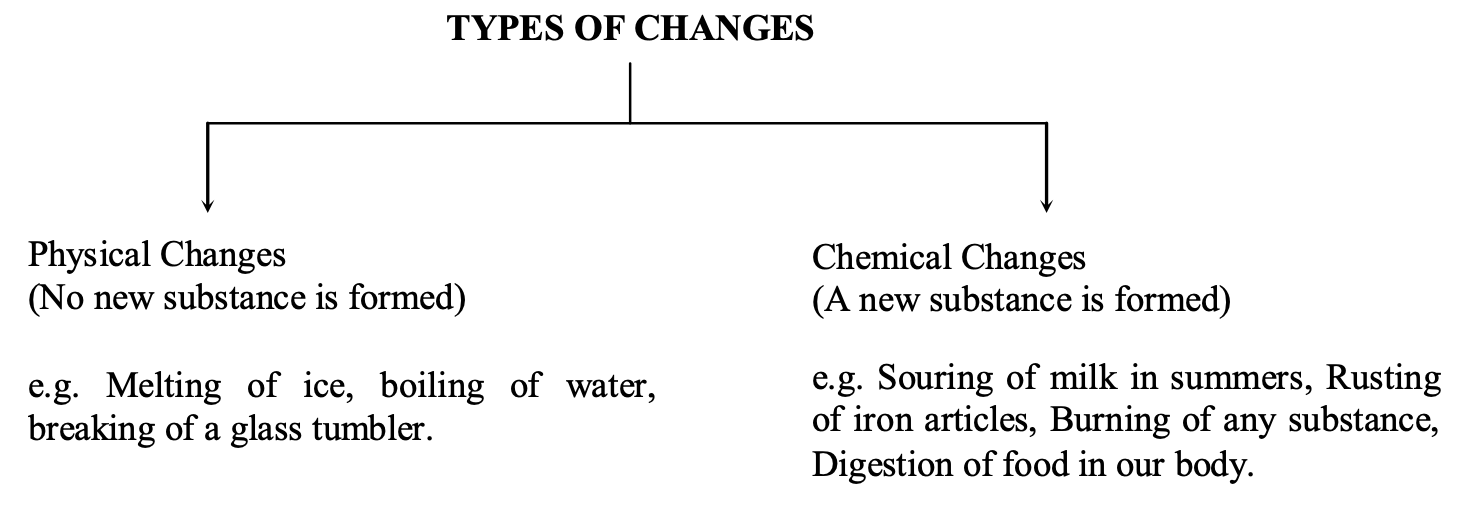
DIFFERENCE BETWEEN PHYSICAL CHANGE AND CHEMICAL CHANGE
| Physical Change |
Chemical Change |
| (i) Those changes in which no new substances are formed are called physical changes. | (i) Those changes in which the original substances lose their chemical nature and identity and form new chemical substances with different properties are called chemical changes. |
| (ii) It is a temporary change. | (ii) It is a permanent change. |
| (iii) It is easily reversible. | (iii) It is usually irreversible. |
| (iv) In a physical change, the mass of the substance does not alter. | (iv) In a chemical change, the mass of the substance does alter |
CHEMICAL REACTION
The process involving a chemical change is called a chemical reaction.
or
A chemical reaction is a process which transforms one or more substances into new substances.
or
The process in which a substance or substances undergo change, to produce new substances with new properties, is known as chemical reaction.
Reactants: The substances which take part in a chemical reaction are called reactants.
Products: The new substances formed as a result of the chemical reaction are called products.
For example
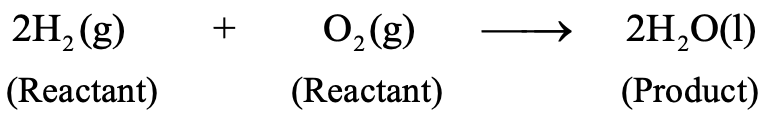
In the above chemical reaction hydrogen and oxygen which are written on the left hand side are reactants and water which is written on the right hand side is a product.
CHARACTERISTICS OF A CHEMICAL REACTION:
When we heat sugar crystals they melt and on further heating they give steamy vapour, leaving behind brownish black mass. On cooling no sugar crystals appears. Thus change which takes place on heating sugar is a chemical change and the process which brings about this chemical change is called chemical reaction.
- In this reaction the substances which take part in bringing about chemical change are called reactants.
- The substances which are produced as a result of chemical change are called products.
- These reactions involve breaking and making of chemical bonds.
- Product(s) of the reaction is/are new substances with new name(s) and chemical formula.
- It is often difficult or impossible to reverse a chemical reaction.
- Properties of products formed during a chemical reaction are different from those of the reactants.
- Apart from heat other forms of energies are light and electricity which are also used in carrying out chemical changes.
In all chemical reactions, the transformation from reactants to products is accompanied by various characteristics, which are
Some chemical reactions are characterized by evolution of a gas.
When zinc metal is treated with dilute sulphuric acid, hydrogen gas is evolved. The hydrogen gas burns with a pop sound.
Zn (s) + H₂SO₄ (dilute) → ZnSO₄ (aq) + H₂(g)
When washing soda is treated with hydrochloric acid, it gives off colorless gas with lots of effervescence.
Na₂CO₃(s) + 2HCl → 2NaCl (aq) + H₂O(l) + CO2(g)
2NaHCO₃ (s) ――heat→ Na₂SO₃ (s) + H₂O(ℓ) + CO₂ (g)
Sodium hydrogen carbonate Sodium carbonate Water Carbon dioxide
Change of colour: Certain chemical reactions are characterized by the change in colour of reacting substance.
When red lead oxide is heated strongly it forms yellow coloured lead monoxide and gives off oxygen gas.
2Pb₃O₄(s) —heat —> 6PbO(s) + O₂(g)
Lead oxide Lead monoxide (Red) (Yellow)
When copper carbonate (green) is heated strongly it leaves behind a black residue.
CuCO₃(s) —heat —> CuO(s) + CO₂(g)
Copper carbonate Copper oxide Carbon dioxide (Green) (Black)
2Pb(NO₃)₂(s) — heat —> 2 PbO(s) + 4NO₂(g) + O₂(g)
Lead (II) nitrate Lead (II) oxide Nitrogen dioxide (White) (Yellow) (Brown)
C₁₂H₂₂O₁₁(s) — heat —> 12C(s) + 11H₂O
White sugar Carbon Black Water
Formation of precipitate:
Some chemical reactions are characterized by the formation of precipitate (an insoluble substance), when the solutions of the soluble chemical compounds are mixed together.
➢ When silver nitrate solution is mixed with a solution of sodium chloride.
AgNO₃ (aq) + NaCl (aq) ⟶ NaNO₃ (aq) + AgCl (s)
Silver nitrate Sodium chloride Sodium nitrate Silver chloride (Colourless) (Colourless) (Colourless) (White precipitate)
A dirty green precipitate of ferrous hydroxide is formed, when a solution of ferrous sulphate is mixed with sodium hydroxide solution.
➢ FeSO₄ (aq) + 2NaOH(aq) ⟶ Na₂SO₄ (aq) + Fe(OH)₂ (aq)
Ferrous sulphate sodium hydroxide Sodium sulphate Ferrous hydroxide (Light green) (Colourless) (Colourless) (Dirty green precipitate)
➢ BaCl₂ (aq) + dill H₂SO₄ ⟶ BaSO₄ (s) + 2HCl (aq)
Barium chloride Barium sulphate (White precipitate)
Energy changes:
All chemical reactions proceed either with the absorption or release of energy.
On the basis of energy changes, there are two types of reactions:
(A) Endothermic reaction: A chemical reaction which is accompanied by the absorption of heat energy is called an endothermic reaction.
C (s) + 2S (s) --Heat→ CS₂ (l)
➢ Light energy is essential for biochemical reaction, photosynthesis, by which green plants prepare their food from carbon dioxide & water.
(B) Exothermic reaction: A chemical reaction which is accompanied by the release of heat energy is called exothermic reaction.
When magnesium wire is heated from its tip in a bunsen flame, it catches fire and burns with a dazzling white flame with release of heat and light energy.
2Mg (s) + O₂ (g) - Heat -> 2MgO (s) + Energy
➢ When quick lime (calcium oxide) is placed in water, the water becomes very hot and sometimes starts boiling. It is because of release of heat energy during the reaction.
CaO (s) + H₂O -> Ca(OH)₂ (aq) + Heat energy Calcium oxide Water Calcium hydroxide
Change of state: Some chemical reactions are characterised by a change in state i.e. solid, liquid or gas
➢ Two volumes of hydrogen gas reacts with one volume of oxygen gas to from water.
2H₂ (g) + O₂ (g) → 2H₂O (ℓ)
or when electric current is passed through water it splits into its elements.
2H₂O(ℓ) -Electric current -> 2H₂ (g) + O₂ (g)
NH₃ (g) + HCl (g) -> NH₄Cl (s) Ammonia Hydrochloric acid Ammonium Chloride
EXAMPLES OF SOME CHEMICAL REACTIONS:
The burning of magnesium in air to form magnesium oxide
Take a magnesium ribbon and clear it by rubbing with a sand paper. Hold it with a pair of tongs. Burn it using a spirit lamp or burner and collect the white ash so formed in a watch-glass as shown in figure.
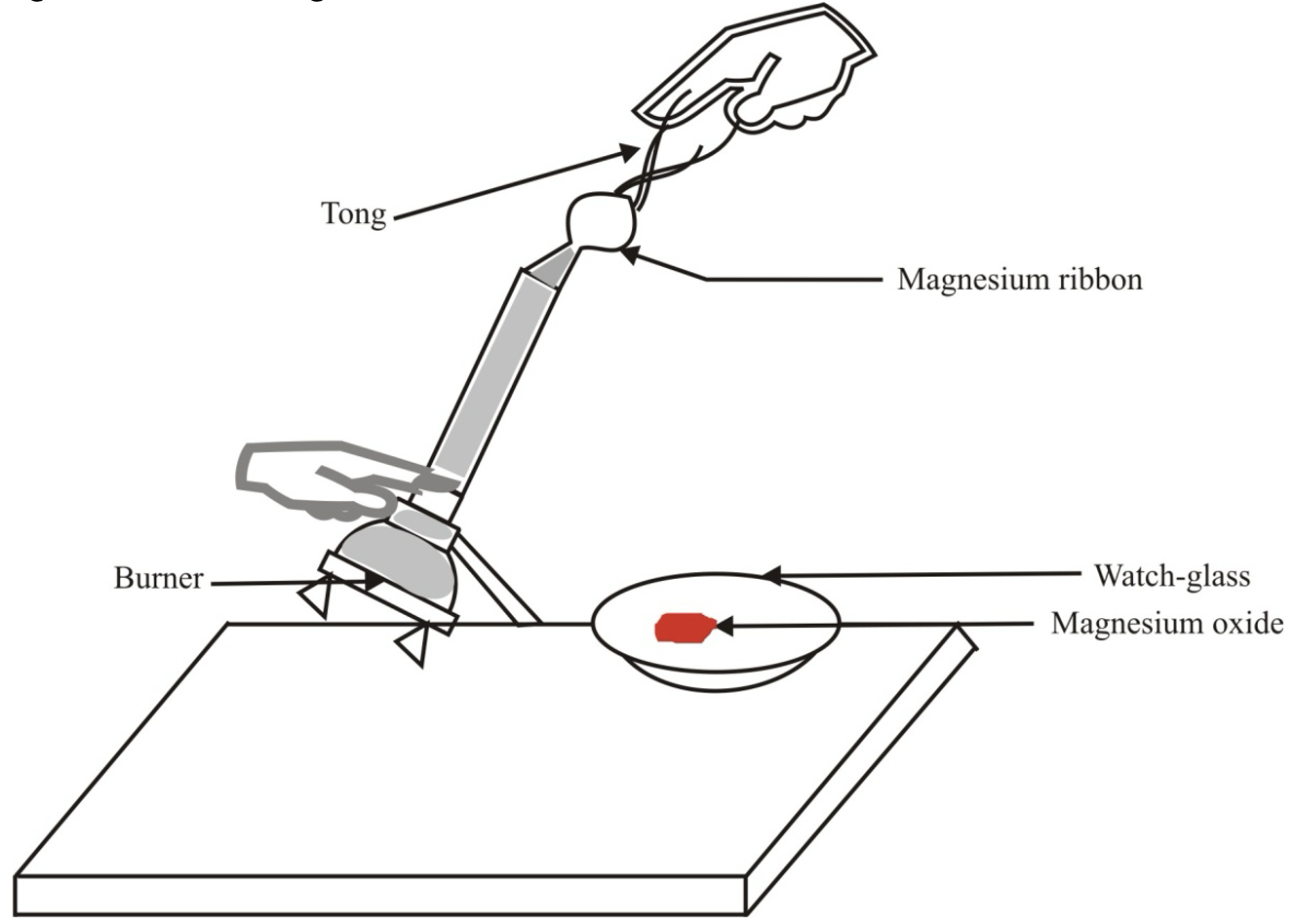
Burning of a magnesium ribbon in air and collection of magnesium oxide in a watch-glass
Magnesium ribbon burns with dazzling light and a white substance is formed which is magnesium oxide. This happens due to the following chemical reaction :
2 Mg (s) + O2 (g) → 2 MgO (s)
Magnesium Oxygen (from air) Magnesium oxide
Thus, a chemical reaction has taken place in which magnesium has combined with oxygen of the air to form a new chemical substance, magnesium oxide (MgO). Here Mg and O2 are reactants and MgO formed is product.
Reaction between lead nitrate and potassium iodide:
Take lead nitrate solution in a test tube and add some potassium iodide solution to this.
A yellow solid namely lead iodide in the form of precipitate appears. Another substance namely potassium nitrate is also formed which we cannot see as it remains in the solution.
This happens due to the following chemical reaction:
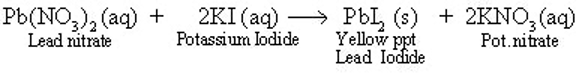
In this reaction lead nitrate and potassium iodide are the reactants while lead iodide and potassium nitrate are the products.
Reaction between zinc and dilute sulphuric acid (or hydrochloric acid)
Take a few zinc granules in a conical flask and add some dilute hydrochloric acid (HCl) or sulphuric acid (H2SO4) to this. A gas is evolved very briskly. If we touch the flask, it is found to be hot.
On bringing a lighted candle near the upper end of the tube fitted in the flask (figure) the gas burns with a popping sound. This confirms that the gas evolved is hydrogen.
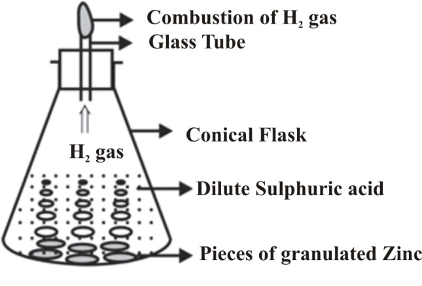
During this reaction, zinc sulphate or zinc chloride is also formed which we cannot see as it remains in the solution. The chemical reactions takes place as follows :
With HCl
Zn + 2HCl ZnCl2 + H2 ↑
Zinc chloride
With H2SO4
Zn + H2SO4 ZnSO4 + H2 ↑
Zinc sulphuric Zinc sulphate
acid
In this chemical reaction, zinc and hydrochloric acid (or sulphuric acid) are the reactants while hydrogen gas and zinc chloride (or zinc sulphate) are the products.
CHEMICAL EQUATIONS
A chemical equation is a symbolic representation of an actual chemical change.
or
The short-hand method of representing a chemical reaction in terms of symbols and formulae of the different reactants and products is called a chemical equation.
A chemical reaction can be represented in two different ways :
STEPS FOR WRITING A CHEMICAL EQUATION:
Writing of a chemical equation involves the following steps :
(i) The symbols and formulae of the reactants are written on the left hand side with plus (+) sign between them.
(ii) The symbols and formulae of the products are written on the right hand side with + sign between them.
(iii) An arrow sign (→) is put between the reactants and the products, pointing from reactants towards products.
Word equations: A word equation links together the names of the reactants with those of the products. For example, the word equation, when magnesium ribbon burns in oxygen to form a white powder of magnesium oxide, may be written as follows-
Magnesium + Oxygen → Magnesium oxide
(Reactants) (Product)
Similarly, the word equation for the chemical reaction between granulated zinc and hydrochloric acid may be written as -
Zinc + Sulphuric acid → Zinc sulphate + Hydrogen
In a word equation:
- The reactants are written on the left hand side with a plus sign (+) between them.
- The products are written on the right hand side with a plus sign (+) between them.
- An arrow (→) separates the reactants from the products.
- The direction of the arrow head points towards the product.
Symbol equation: A brief representation of a chemical reaction in terms of symbols and formulae of the substances involved is known as a symbol equation.
In a symbol equation, the symbols and formulae of the elements and compounds are written instead of their word names.
For e.g. Burning of magnesium in oxygen to form magnesium oxide may be written as
follows:
Mg + O2 → MgO
BALANCED AND UNBALANCED CHEMICAL EQUATIONS:
Balanced chemical equation:
The equation, in which the number of atoms of each element in the reactants, and the products sides are equal, is called a balanced chemical equation.
The chemical equations are balanced to satisfy the law of conservation of mass in chemical reactions.
For example, In a chemical reaction between zinc and dilute sulphuric acid are reactants, giving zinc sulphate and hydrogen as products. The chemical equation can be written as
Zn + H2SO4 → ZnSO4 + H2.
Here the numbers of atoms of each element in the reactant and products sides are equal. i.e.
In reactants In products
| No. of Zn atoms | 1 | 1 |
| No. of H atoms | 2 | 2 |
| No. of S atoms | 1 | 1 |
| No. of O atoms | 4 | 4 |
Hence it is a balanced chemical equation.
Unbalanced chemical equation (skeletal equation):
The equation in which the number of atoms of different elements on the reactants and the product sides are not equal is called an unbalanced chemical equation. The unbalanced chemical equation is also known as skeletal equation.
For example
The burning of aluminium in oxygen to form aluminium oxide can be written as :
Al + O2 → Al2 O3
Here the number of atoms of each element in the reactants and products side is not equal. i.e.
| In reactants |
In products |
|
| No. of Al atoms | 1 | 2 |
| No. of O atoms | 2 | 3 |
Hence it is an unbalanced chemical equation.
BALANCING A CHEMICAL EQUATION:
Balancing of chemical equations may be defined as the process of making the number of different types of elements, on both side of the equations, equal.
The balancing of a chemical equation is done with the help of Hit and Trial method. In this method, the coefficients before the symbols or formulae of the reactants and products are adjusted in such a way that the total number of atoms of each element on both the sides of the arrow head become equal. This balancing is also known as mass balancing because the atoms of elements on both side are equal and their masses will also be equal.
The major steps involved in balancing a chemical equation are as follow –
- Write the chemical equations in the form a word equations. Keep the reactants on the left side and the products on the right side. Separate them by an arrow whose head points from the reactants towards the product.
- Convert the word equation into the symbol equation by writing the symbols and formulae of all the reactants and product.
- Make the atoms of different elements on both side of the equation equal by suitable method. This is known as balancing of equation.
- Do not change the formulae of the substance while balancing the equation.
- Make the equations more informative if possible.
Writing State Symbols:
The chemical equations or symbol equations which we have enlisted don't mention the physical states of the reactant and product species involved in the reaction. In order to make the equation more informative, the physical state are also mentioned with the help of certain specific symbols known as state symbols. These symbols are
- (s) for solid state
- (ℓ) for liquid state
- (g) for gaseous state
- (aq) for aqueous solution i.e., solution prepared in water.
Sometimes a gas if evolved in a reaction is shown by the symbol (↑) i.e., by an arrow pointing upwards. Similarly the precipitate, if formed during the reaction, is indicated by the symbol (↓) i.e., by an arrow pointing downwards.
The abbreviation 'ppt' is also used to represent the precipitate, if formed.
(i) 2Na(s) + 2H₂O(ℓ) → 2NaOH(aq) + H₂(g) or H₂(↑)
(ii) Ca(OH)₂(aq) + CO₂(g) → CaCO₃(↓) + H₂O(ℓ)
(iii) AgNO₃(aq) + NaCl(aq) → AgCl(↓) + NaNO₃(aq)
Significance of State Symbols:
The state symbols are of most significance for those chemical reactions which are either accompanied by the evolution of heat (exothermic) or by the absorption of heat (endothermic). For example:
2H₂(g) + O₂(g) → 2H₂O(ℓ) + 572 kJ
2H₂(g) + O₂(g) → 2H₂O(g) + 44 kJ
Both these reactions are of exothermic nature because heat has been evolved in these. However actual amounts of heat are different when water is in the liquid state i.e. H₂O(ℓ) and when it is in the vapour state.
Specialities of Chemical Equation:
(i) We get the information about the substance which are taking part and formed in the reaction.
(ii) We get the information about the number of molecules of elements or compounds which are either taking part or formed in the chemical reaction.
(iii) We also get the information of weight of the reactants or products.
For example: CaCO₃ ⟶ CaO + CO₂ (100gm) (56 gm) (44 gm)
Total weight of the reactants is equal to the total weight of the products because matter is never destroyed. In the above example total weight of calcium carbonate (reactant) is 100 gram and of product is also 100 g (56 gram + 44 gram).
(iv) In a chemical equation if any reactant or product is in gaseous state, then its volume can also be determined. For example in the above reaction volume of carbon dioxide is 22.4 liters.
(v) In a chemical equation with the help of the product we can get information about the valency as well.
For example:
Mg + 2HCl → MgCl₂ + H₂(↑)
In the above reaction one atom of Mg displaces two atoms of hydrogen, so valency of magnesium is two.
LIMITATIONS OF A CHEMICAL EQUATION:
It gives us no information about the following :
- The physical state of the reactants.
- The concentration of the reactants.
- The time taken for the reaction to complete.
- The rate at which the reaction proceeds.
- The conditions necessary to start and carry on the reaction e.g., Is any catalyst required? What is the temperature needed to start and continue the reaction?
- Is the reaction exothermic or endothermic, i.e, is heat evolved or absorbed during the reaction?
ESSENTIALS OF A CHEMICAL EQUATION:
A true chemical equation, therefore, must be in accordance with the following essentials :
- It should represent an actual chemical change.
- It should be balanced, i.e., number of atoms of different elements on the two sides of the equation must be equal.
- It should be molecular i.e., all the substances concerned should be expressed as molecules.
TO MAKE CHEMICAL EQUATION MORE INFORMATIVE:
It is quite helpful if an equation gives an idea about the physical state, heat changes and the conditions under which the reaction takes place.
This can be done in the following three ways.
To indicate the physical states of reactants and products:
The physical states of the reactants and products of a chemical reactions can be indicated by writing letters (g) for gaseous state, (l) for liquid state, (s) for solid state and (aq) for aqueous solutions i.e. solutions in water, just after the formulae in an equation as shown in the examples below:
Zn(s) + H₂SO₄(aq) → ZnSO₄(aq) + H₂(g)
➤ An arrow pointing upwards (↑) may also be used to indicate a gaseous product in the equation as follows:
Zn(s) + H₂SO₄(aq) → ZnSO₄(aq) + H₂↑.
➤ An arrow pointing downwards (↓) is used to indicate an insoluble product or precipitate (ppt) in an equation as follows:
AgNO₃(aq) + NaCl(aq) → AgCl(s)↓ + NaNO₃(aq)
Silver nitrate + Sodium chloride → Silver chloride + Sodium nitrate
(Precipitate)
To indicate the heat changes (thermochemical equations):
Chemical equations representing the heat evolved or absorbed during the reaction are called thermochemical equations.
(a) Exothermic reaction (heat evolved): An exothermic reaction is indicated by writing + heat, or + heat energy or + energy, on the products side of an equation as follows:
C(s) + O₂(g) → CO₂(g) + heat
CaO(s) + H₂O(l) → Ca(OH)₂ + heat
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g) + heat
(b) Endothermic reaction (heat absorbed): An endothermic reaction is indicated by writing "+ heat" or "+ heat energy" or "+ energy" on the reactants side of an equation as shown below :
CaCO₃(s) + heat → CaO(s) + CO₂(g) Calcium carbonate Calcium oxide Carbondioxide
N₂(g) + O₂(g) + heat → 2NO(g) Nitrogen Oxygen Nitric oxide
Ba(OH)₂ + 2 NH₄Cl + heat → BaCl₂ + 2 NH₄OH Barium hydroxide Ammonium chloride Barium chloride Ammonium hydroxide
To indicate the conditions under which the reaction takes place
The conditions of temperature, pressure and the presence of catalyst if any are represented by writing these conditions above or below the arrow sign as follows:
N₂(g) + 3H₂(g) → 2NH₃(g) Nitrogen Hydrogen Ammonia 500°C, 200atm Fe
Here 500°C temperature, 200 atm pressure and Fe as catalyst are the condition for the reaction to take place.
CO(g) + 2H₂(g) → CH₃OH(l) Carbon Hydrogen Methanol monooxide 300°C, 300atm ZnO+CrO₃
Here 300°C temperature, 300 atm pressure and zinc oxide (ZnO) and chromium oxide CrO₃ as catalyst are the conditions needed for the reaction to take place.
If heat is required for the reaction to take place, then heat sign delta (Δ) is written over the arrow of the equation as follows:
2KClO₃(s) → 2KCl(s) + 3O₂(g) Potassium chlorate Potassium chloride Oxygen Δ MnO₂
Here Δ shows the heat needed and MnO₂ is the catalyst needed for the reaction to take place.
TYPES OF CHEMICAL REACTIONS
COMBINATION REACTIONS:
Those reactions in which two or more substances (reactants) combine together to form a single substance (product) are called the combination reactions.
Ex 1: Formation of water from H₂(g) and O₂(g)
2H₂(g) + O₂(g) → 2H₂O(l) Hydrogen Oxygen Water
In this reaction, two substances hydrogen and oxygen (reactants) combine together to form a single substance i.e. water (product). So it is a combination reaction.
Synthesis reaction: It is a type of addition reaction in which a new substance is formed by the union of its component elements.
For e.g. N₂ + 3H₂ ⟶ 2NH₃ (Haber's Process)
Ammonia is synthesised from its components, nitrogen and hydrogen, so it is a synthetic reaction. All synthesis reactions are addition reactions
Other examples of synthesis reactions are:
(i) CaO(s) + H₂O(g) ⟶ Ca(OH)₂(aq)
C(s) + O₂(g) ⟶ CO₂(g)
(ii) When two or more compounds combine to form a new compound For e.g.
NH₃ + HCl ⟶ NH₄Cl
CH₂ = CH₂ + Br ⟶ CH₂ - Br
|
CH₂-Br
(iii) When an element and a compound combine to form a new compound.
2CO + O₂ ⟶ 2CO₂
DECOMPOSITION REACTIONS:
Those reactions in which a single substance (reactant) splits up into two or more simpler substances (products) are known as decomposition reactions.
These reactions are carried out by supplying energy in form of heat, electricity or light which breaks that substance into simpler substances. Thus decomposition reactions are classified as:
- Thermolysis or thermal decomposition reactions (decomposition by heat).
- Electrolysis or electrolytic decomposition reactions (decomposition by electricity)
- Photolysis or photodecomposition reactions (decomposition by light).
Decomposition reactions are called the opposite of combination reactions
In a decomposition reaction, one substance breaks up into two or more chemical substances, while in a combination reaction two or more substances combine to form one single substance. So these two reactions are called opposite of each other.
Uses of Decomposition Reactions
The decomposition reactions are used in the extraction of metals in the following ways :
(i) Metals are extracted from their molten salts by electrolytic decomposition e.g. sodium from molten sodium chloride and aluminium from alumina (molten aluminium oxide).
(ii) Thermal decomposition reactions form one of the steps in extraction of metals.
For example, Zinc carbonate (the naturally occurring ore of zinc) is first decomposed to give zinc oxide and then reduced to obtain zinc metal i.e.,
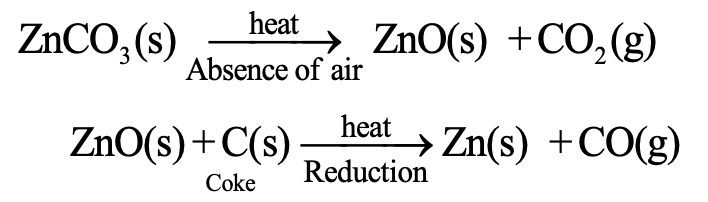
Decomposition Reactions in our body:
The digestion of food in the body is an example of decomposition reaction. When we eat foods like wheat, rice or potatoes, then the starch present in them decomposes to give simple sugars like glucose in the body and proteins decompose to form amino acids :
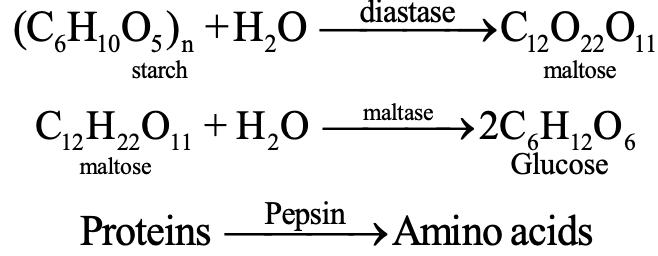
Decomposition Reactions are endothermic reactions:
All decomposition reactions require energy either in form of heat, light or electricity. Hence all decomposition reactions are endothermic (heat absorbing) reactions.
DISPLACEMENT REACTIONS:
It involves displacement of one of the constituents of a compound by another substance and may be regarded as a displacement reaction.
Relative activities or reactivities of metals:
Metals have been arranged in decreasing order of their activities (or reactivities) in the activity series as follows:
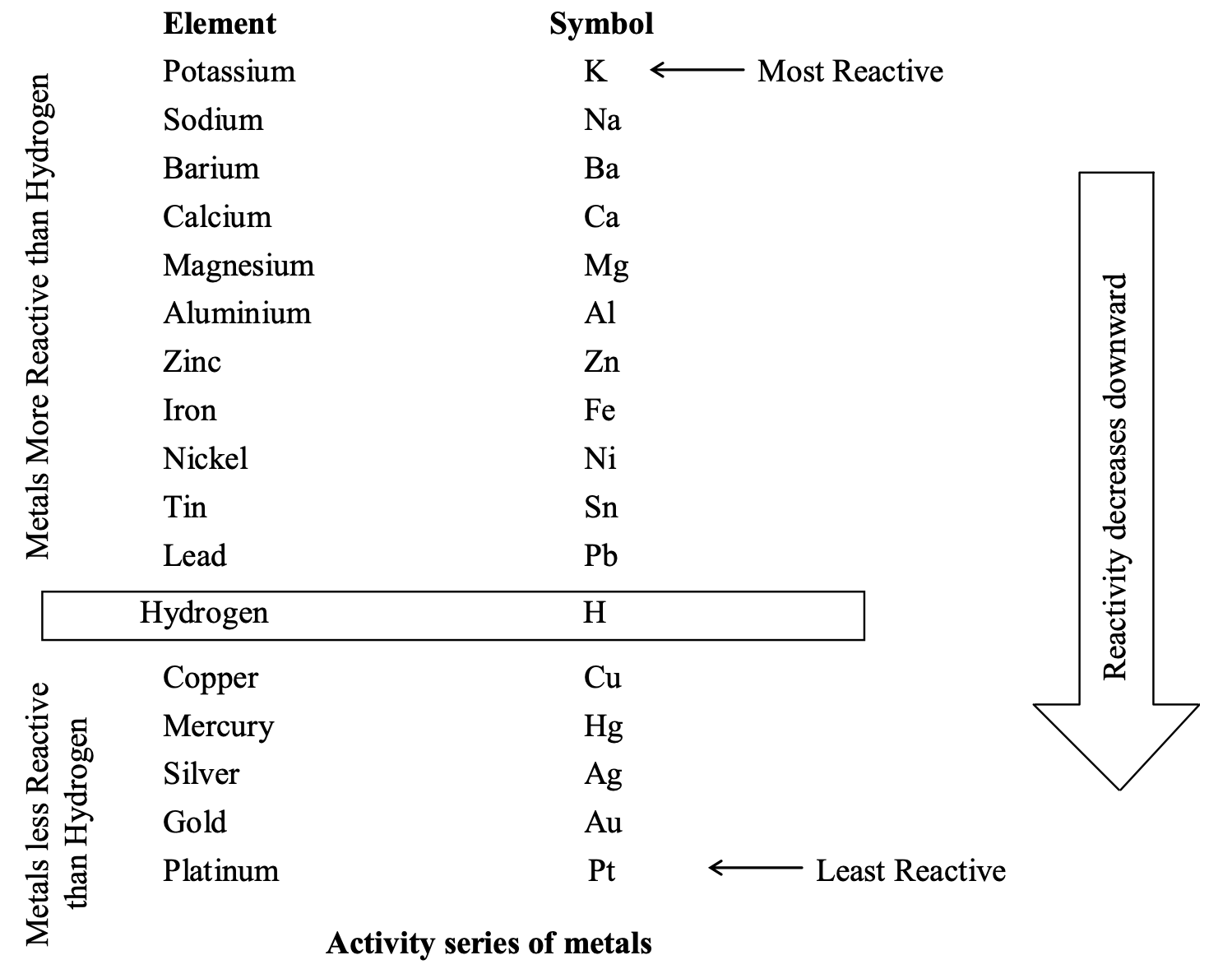
It is clear from the series that the metals lying above the hydrogen are more reactive than the metals lying below the hydrogen. Thus, any metal can displace the metals lying below it from its solution.
Relative activities (or reactivities) of Non-metals:
Relative activities of non-metals like halogens is in the order:
F > Cl > Br > I
Thus, fluorine is most reactive and iodine is least reactive. So fluorine (F2) can displace chlorine (Cl2) from NaCl, Bromine (Br2) from NaBr and so on. Similarly, chlorine can displace bromine (Br2) from KBr and iodine (I2) from KI and so on.
Displacement reactions in which a more reactive metal displaces a less reactive metal from its compound:
Example:
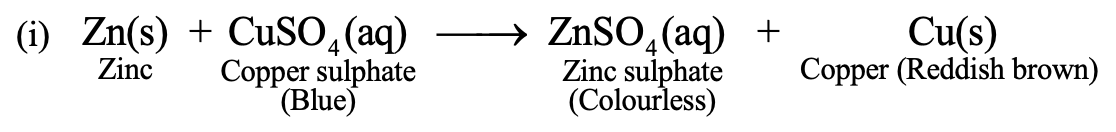
Thus, when zinc pieces are added to copper sulphate solution, then, zinc being more reactive metal than copper, displaces copper from its solution (CuSO4) so that Cu is set free. The blue colour of CuSO4 solution fades due to formation of ZnSO4 (colourless). A reddish brown deposit of copper metal is formed on the surface of zinc. Therefore it is an example of displacement reaction.
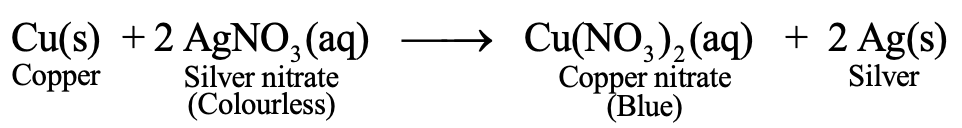
When a copper wire is dipped in silver nitrate solution, copper, being more reactive metal than silver, displaces silver from its solution (AgNO3) so that silver is liberated. This silver is deposited on the copper wire giving it a white shining surface. The solution forms a blue colour due to formation of copper nitrate. Thus it is an example of displacement reaction.

In this reaction sodium, being more active than hydrogen, displaces hydrogen from water so that hydrogen gas is liberated along with the formation of sodium hydroxide. Thus, it is an example of displacement reaction.
(b) The displacement reactions in which more reactive non-metal displaces less reactive non-metal from its compound:
e.g.
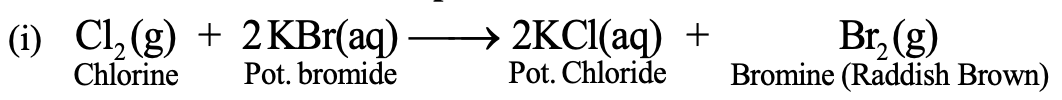
When Cl2 gas is passed through an aqueous solution of potassium bromide, chlorine being more reactive than bromine, displaces bromine from KBr so that bromine gas (Br2) is liberated. The solution forms light brown colour due to dissolution of Br2 gas in it. So it is an example of displacement reaction.
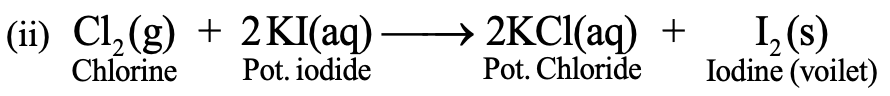
When chlorine gas is passed through potassium iodide solution, chlorine, being more reactive than iodine, displaces iodine from KI and liberates I2 gas.
The solution acquires violet colour due to dissolution of I2 gas in it. So, it is also an example of displacement reaction.
Uses of Displacement Reactions:
Displacement reactions are used in the extraction of silver and gold. Silver or gold ore is dissolved in sodium cyanide solution. When zinc granules are added to the solution of the compound formed, zinc, being more active than silver and gold, displaces silver and gold from the solution of their compounds and thus silver and gold are extracted.
Displacement reactions are exothermic:
All displacement reactions are exothermic (heat producing) reactions, For example :
- In the displacement reaction between zinc and dilute hydrochloric acid or dilute sulphuric acid, there is production of heat along with evolution of gas.
- In displacement reaction between iron and copper sulphate solution, there is an increase in temperature due to production of heat. Thus, displacement reactions are heat producing or exothermic reactions.
DOUBLE DISPLACEMENT REACTIONS
Those reactions in which two different atoms or groups of atoms are exchanged are called the double displacement reactions. These reactions generally occur between two ionic compounds in the solution. So they may be defined as :
“Those reactions in which two ionic compounds in the solution react by exchange of their ions to form new compounds are called double displacement reactions”
OXIDATION – REDUCTION REACTIONS OR REDOX REACTIONS
OXIDATION:
Oxidation is a chemical reaction in which a substance gains oxygen or loses hydrogen. Since oxygen is an electronegative element and hydrogen is an electropositive element, so, oxidation is defined as a reaction in which a substance gains an electronegative radical or loses an electropositive radical.
Oxidation can be defined in three ways:
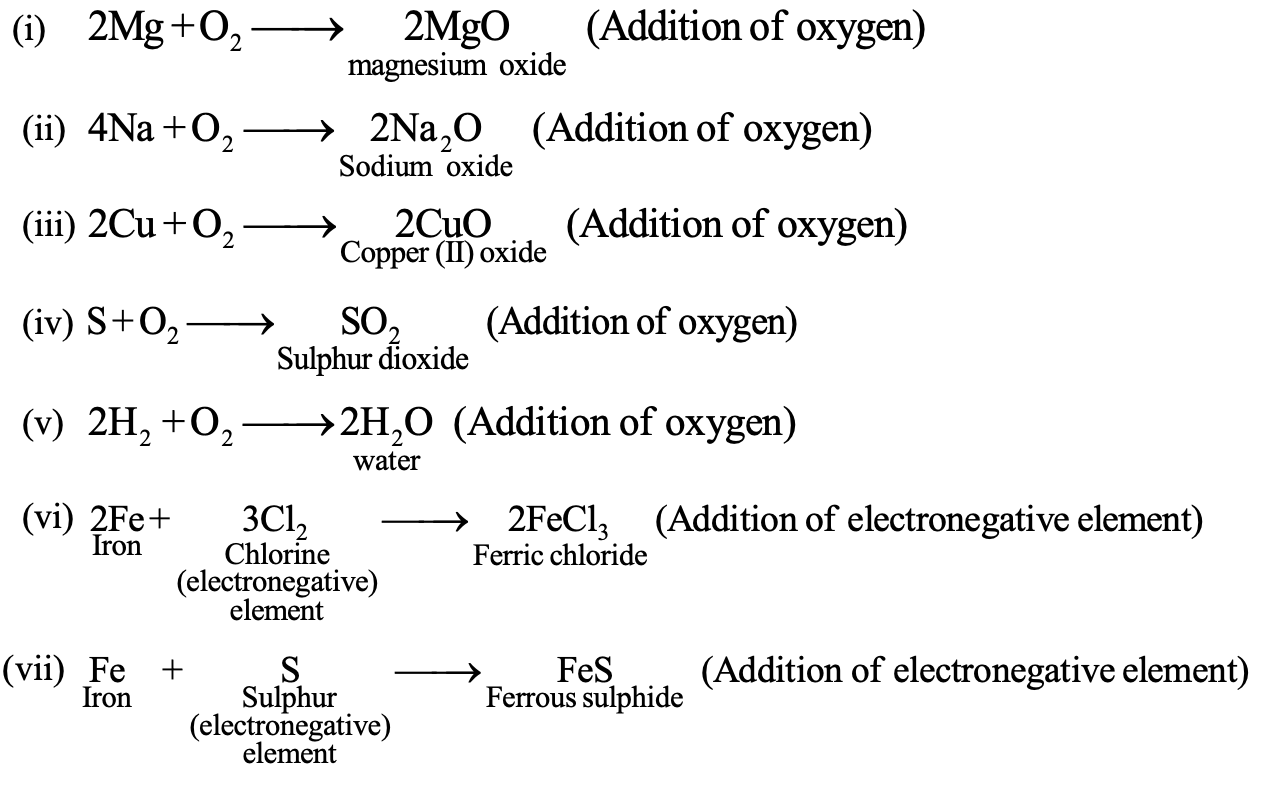
(1) Oxidation in terms of oxygen or electronegative element:
Oxidation is a process in which oxygen or electronegative element is added to a substance. For example:
(2) Oxidation in terms of hydrogen or electropositive element
Oxidation is defined as a process in which hydrogen or an electropositive element is removed from the substance i.e.
Removal of hydrogen
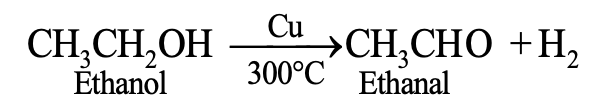
(3) Oxidation in terms of electronic concept
Oxidation is a process in which loss of electrons take place.
As a result, there is increase in positive charge or decrease in negative charge of the atom or group of atoms in an oxidation reaction e.g.,
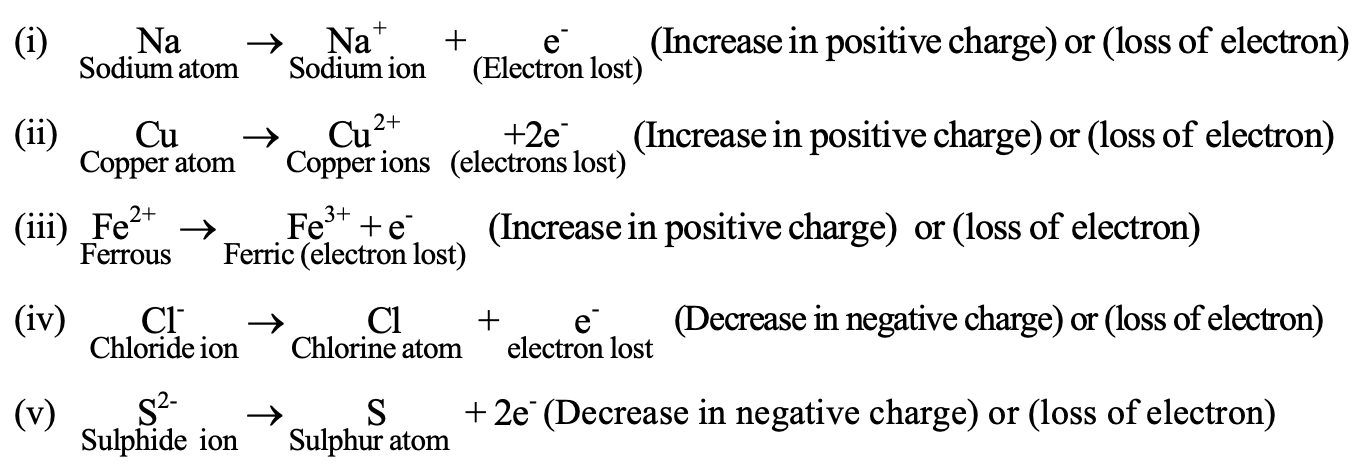
REDUCTION:
It is a chemical reaction in which there is a gain of hydrogen or any electropositive radical or a loss of oxygen or electronegative radical.
Reduction can also be defined in three ways:
(1) Reduction in terms of oxygen or electronegative element: Reduction is a process in which oxygen or electronegative element is removed from a substance. For Example:
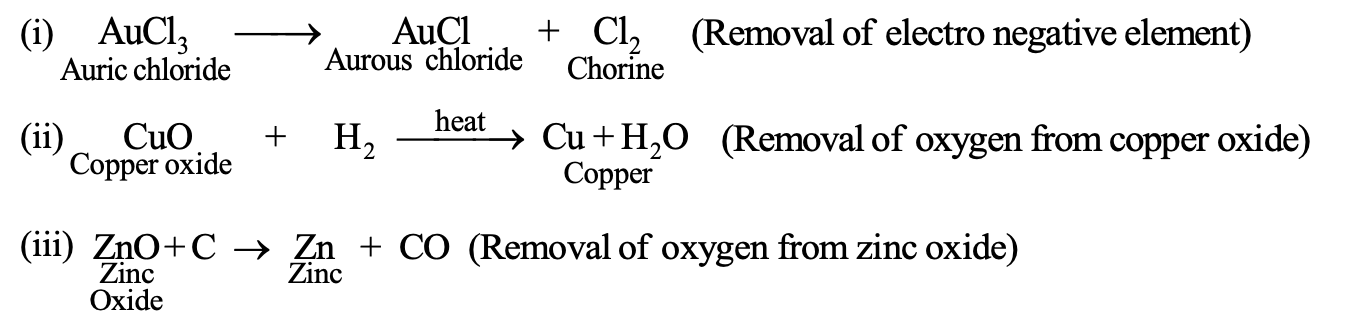
(2) Reduction in terms of hydrogen or electropositive element: Reduction is a process in which hydrogen or electropositive element is added to a substance. For Example:
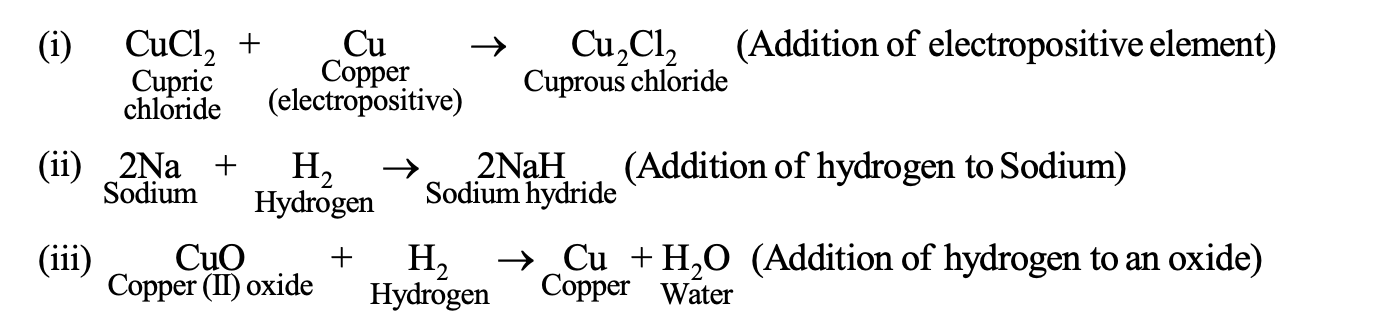
(3.)Reduction in terms of electronic concept : Reduction is a process in which gain of electrons takes place.
or
Reduction is a process in which electrons are gained by an atom, ion or a group of atoms. As a result, there is an increase in negative charge or decrease in positive charge. Example:
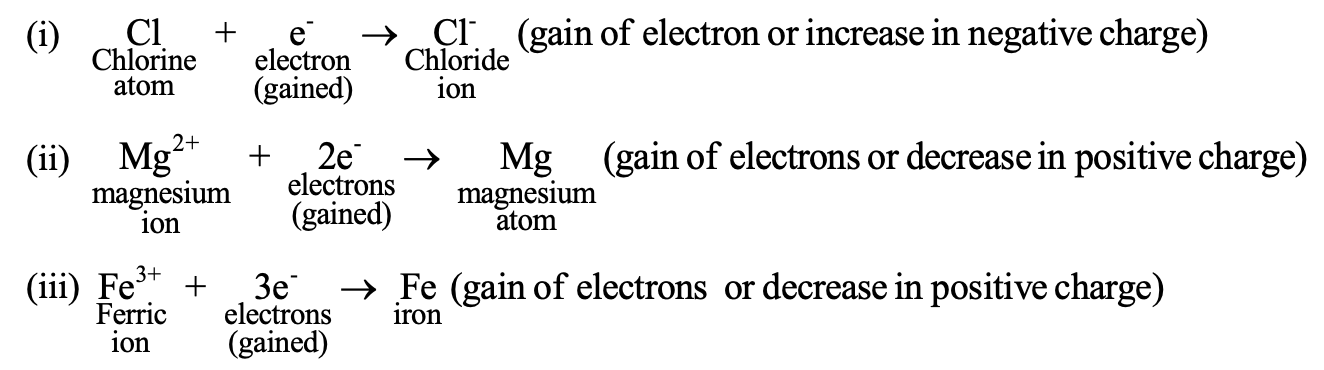
REDOX REACTIONS:
Reduction is loss of electronegative element or radical. From all above example it is clear that oxidation and reduction occur side by side, i.e. there can be no oxidation without an equivalent reduction. In a reaction whenever one substance is oxidised the other is definitely reduced. The reverse is also true whenever one substance is reduced the other is oxidised. Such reactions in which oxidation and reduction take place simultaneously are known as redox reactions.
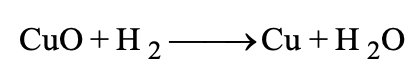
MOLECULAR AND IONIC EQUATIONS:
(1) Molecular equations: When the reactants and products involved in a chemical change are written in molecular forms in the chemical equation, it is termed as molecular equation.
Example:
In above example the reactants and products have been written in molecular forms, thus the equation is termed as molecular equation.
(2) Ionic equations: When the reactants and products involved in a chemical change are ionic compounds, these will be present in the form of ions in the solution. The chemical change is written in ionic forms in chemical equation, it is termed as ionic equation. Example:
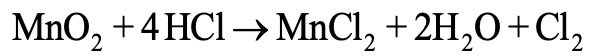
In above example the reactants and products have been written in ionic forms, thus the equation is termed as ionic equation.
(3) Spectator ions: In ionic equations, the ions which do not undergo any change and equal in number in both reactants and products are termed as spectator ions and are not included in the final balanced equations. Example,
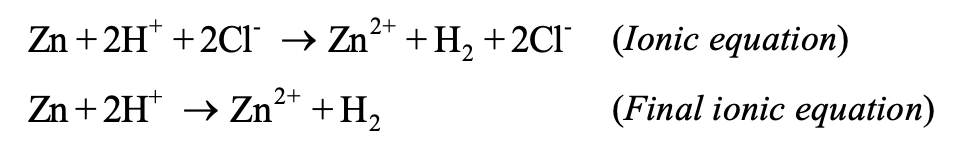
In above example, the Cl- ions are the spectator ions and hence are not included in the final ionic balanced equation.
(a) Oxidizing agent
Oxidation is a process which involves; addition of oxygen, removal of hydrogen, addition of non-metal, removal of metal, Increase in +ve valency, loss of electrons and increase in oxidation number.
(i) Addition of oxygen : 2Mg + O₂ → 2MgO
(ii) Removal of hydrogen : H₂S+Cl₂ → 2HCl + S
(iii) Addition of Non-metal : Fe + S → FeS
(iv) Removal of metal : 2KI+H₂O₂ → 2KOH+I₂
(v) Increase in +ve valency : Fe²⁺ → Fe³⁺ + e⁻
(vi) Loss of electrons (also known as de-electronation)
| -4 | -3 | -2 | -1 | 0 | +1 | +2 | +3 | +4 |
|---|---|---|---|---|---|---|---|---|
| M | M | M | M | M | M | M | M | M |
| -e⁻ | -e⁻ | -e⁻ | -e⁻ | -e⁻ | -e⁻ | -e⁻ | -e⁻ |
Loss of electrons
(a) H⁰ ⟶ H⁺ + e⁻ (Formation of proton)
(b) MnO₄²⁻ ⟶ MnO₄⁻ + e⁻ (De-electronation of MnO₄²⁻)
(c) 2Fe⁰ ⟶ 2Fe³⁺ + 6e⁻ (De-electronation of iron)
(b) Reducing agent
Reduction is just reverse of oxidation. Reduction is a process which involves; removal of oxygen, addition of hydrogen, removal of non-metal, addition of metal, decrease in +ve valency, gain of electrons and decrease in oxidation number.
(i) Removal of oxygen: CuO + C ⟶ Cu + CO
(ii) Addition of hydrogen: Cl₂ + H₂ ⟶ 2HCl
(iii) Removal of non-metal
2HgCl₂ + SnCl₂ ⟶ Hg₂Cl₂ + SnCl₄
(iv) Addition of metal: HgCl₂ + Hg ⟶ Hg₂Cl₂
(v) Decrease in +ve valency
(a) Fe³⁺ ⟶ Fe²⁺ (+ve valency decreases)
(b) [Fe(CN)₆]³⁻ ⟶ [Fe(CN)₆]⁴⁻ (−ve valency increases)
(vi) Gain of electrons (also known as electronation)
-4 -3 -2 -1 0 +1 +2 +3 +4
M M M M M M M M M
+e⁻ +e⁻ +e⁻ +e⁻ +e⁻ +e⁻ +e⁻ +e⁻
Gain of electrons
(a) Zn²⁺(aq) + 2e⁻ ⟶ Zn(S) (Electronation of Zn²⁺)
(b) Pb²⁺ + 2e⁻ ⟶ Pb⁰ (Electronation of Pb²⁺)
(c) [Fe(CN)₆]³⁻ + e⁻ ⟶ [Fe(CN)₆]⁴⁻ (Electronation of [Fe(CN)₆]³⁻)
(vii) Decrease in oxidation number
(a) Mg²⁺ ⟶ Mg⁰ (From +2 to 0)
(b) [Fe(CN)₆]³⁻ ⟶ [Fe(CN)₆]⁴⁻ (From +3 to +2)
(c) Cl₂⁰ ⟶ 2Cl⁻ (From 0 to −1)
ELECTRONIC INTERPRETATION OF OXIDATION:
The electronic theory attempts to interpret oxidation on the basis of electron transfer. According to octet rule, atom will try to complete its octet by losing, gaining or sharing electrons. Sodium chloride is an electrovalent compound and consists of an ion pair (Na+) (CI) even in the solid state. In its formation, the neutral sodium loses an electron and becomes positively charged sodium ion. Sodium is said to be oxidised and loss of electrons is termed as oxidation.
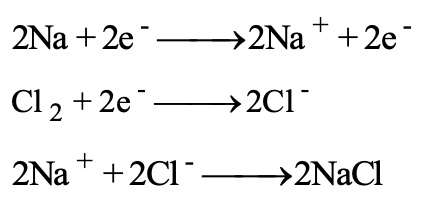
(b) Electronic Interpretation of Reduction:
Reduction which is also referred to as electronation is a process involving the gain of electrons and is the reverse of oxidation.
For example:
Mg combines with oxygen and is oxidised to MgO. According to electronic theory magnesium atom loses two electrons from its outermost shell (M) and is oxidised to Mg while oxygen atom gains these two electrons and gets reduced to oxide anion, hence oxidation involves loss of electrons and it is also referred as de- electronation. Reduction involves gain of electrons so it is referred to as electronation.
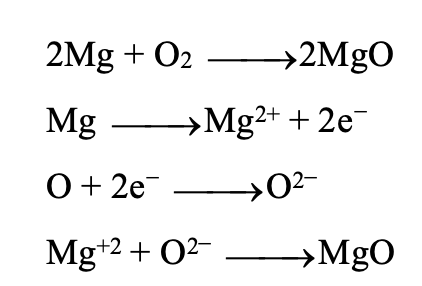
OXIDATION REACTIONS IN EVERYDAY LIFE:
We are all aware of the fact that oxygen is most essential for sustaining life. One can live without food or even water for a number of days but not without oxygen. It is involved in a variety of actions which have wide range of effects on our daily life. Most of them are quite useful while a few may be harmful in nature. Some of these effects are briefly discussed. Some examples are:
(a) Combustion Reactions:
A chemical reaction in which a substance burns or gets oxidised in the presence of air or oxygen is called combustion reaction. For example, kerosene, coal, charcoal, wood etc. burn in air and thus, undergo combustion. Methane (CH4) a major constituent of natural gas undergoes combustion in excess of oxygen upon heating.
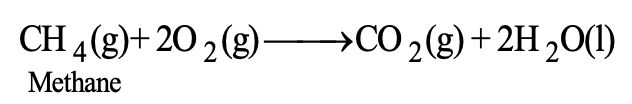
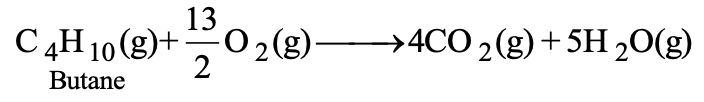
Similarly, butane (C4H10), the main constituent of L.P.G. also undergoes combustion.
All combustion reactions are of exothermic nature and are accompanied by release of heat energy. The human body may be regarded as a furnace or machine in which various food stuffs that we eat undergo combustion or oxidation. The heat energy evolved keeps our body working. Carbohydrates such as glucose, fructose, starch etc. are the major source of energy to the human body. They undergo combustion with the help of oxygen that we inhale to form carbon dioxide and water. For example.
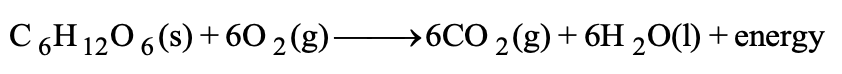
All combustion reactions are not accompanied by flame. Combustion is basically oxidation accompanied by release of energy.
(b) Respiration:
Respiration is the most important biochemical reaction which releases energy in the cells. When we breathe in air, oxygen enters our lungs and passes into thousands of small air sacs (alveoli). These air sacs occupy a large area of membranes and oxygen diffuses from the membranes into blood. It binds itself to haemoglobin present in red blood cells and is carried to millions of cells in the body. Respiration occurs in these cells and is accompanied by the combustion of glucose producing carbon dioxide and water. Since the reaction is of exothermic nature, the energy released during respiration carry out many cell reactions and also keeps our heart and muscles working. It also provides the desired warmth to the body. Both carbon dioxide and water pass back into the blood and we ultimately breathe them out. Respiration takes place in the cells of all living beings.
EFFECTS OF OXIDATION REACTIONS IN EVERYDAY LIFE:
We have discussed the utility of combustion in releasing energy which our body needs to keep warm and working. However, combustion has harmful effects also. The environmental pollution is basically due to combustion. Poisonous gases like carbon monoxide (CO), sulphur dioxide (SO2) sulphur trioxide (SO3) and oxides of nitrogen (NOx) etc. are being released into the atmosphere as a result of variety of combustion reactions which are taking place. They pollute the atmosphere and make our lives miserable. In addition to these, other harmful effects of combustion are corrosion and rancidity. These are briefly discussed.
(i) Corrosion: Corrosion may be defined as the process of slow eating up of the surfaces of certain metals when kept in open for a long time.
Quite often, when we open the bonet of a car after a long time, we find a deposit around the terminals of the battery. This is an example of corrosion. Black coating on the surface of silver and green layer on the surface of coppers is the examples of corrosion. In case of iron, corrosion is called rusting. Rust is a chemical substance brown in mass and is formed by the chemical action of moist air (containing O2 and H2O) on iron. It is basically an oxidation reaction and the formula of rust is Fe2O3. xH2O. It is very slow in nature and once started keeps on.
Both corrosion and rusting are very harmful and cause damage to the buildings, railways tracks, cars and other objects materials where metals are used. We quite often hear that an old building has collapsed on its own causing loss of both lives and property. This is on account of the rusting of iron which is used in making the structure particularly the roof.
Ex. Corrosion of iron (Rusting) :
The most common example of corrosion is rusting i.e., corrosion of iron. When an iron object is left in the moist air for a long time, its surface is covered with a brown, flaky (non-sticky) substance called rust. Rust is mainly hydrated ferric oxide (Fe2O3.xH2O). It is formed due to attack of oxygen and water vapour present in moist air on the surface of iron.
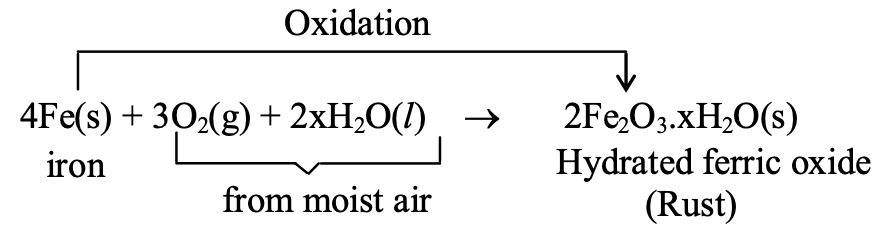
This reaction is called corrosion of iron or rusting.
(i) Effect of Rusting in everyday life : Rusting is a serious problem because it weakens the structure of bridges, iron railings, automobile parts etc. Every year, a lot of money is spent to replace rusted iron and steel structures. The reason is that reddish brown crust of rust does not stick to the surface. It falls down exposing fresh surface for rusting. Thus, corrosion of iron or rusting is a continuous process which ultimately eats up the whole iron object.
(ii)Methods to prevent rusting or Prevention of Rusting: Rusting can be prevented if iron objects are not allowed to come in contact with the moist air. It can be done by :
- Painting the iron objects such as iron gates, steel furniture, bodies of cars etc.
- Greasing and oiling the iron objects such as machine parts etc.
- Galvanisation is coating the surface of iron objects with a thin layer of zinc, which is more resistant to corrosion.
(iii) Some more examples of corrosion of metals
- Corrosion of copper: When a copper object (shiny brown) is left in moist air for a long time, then its surface is covered with green coating of basic carbonate, CuCO3 . Cu(OH)2. This is due to attack of O2, CO2 and water vapour present in moist air on the surface of copper:
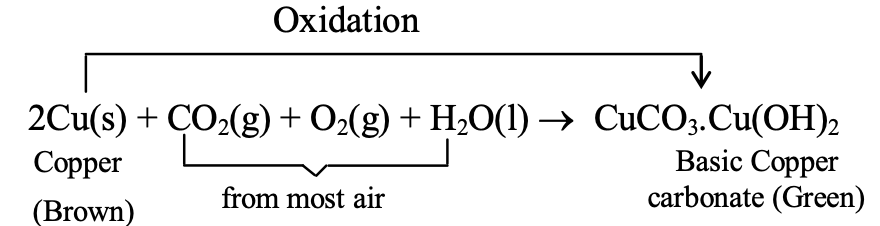
This reaction is called corrosion of copper.
Corrosion of Silver: When a silver object (shiny white) is kept in air for a long time, its surface is covered with coating of black silver sulphide (Ag2S). This is due to the attack of H2S gas present in air on the surface of silver.
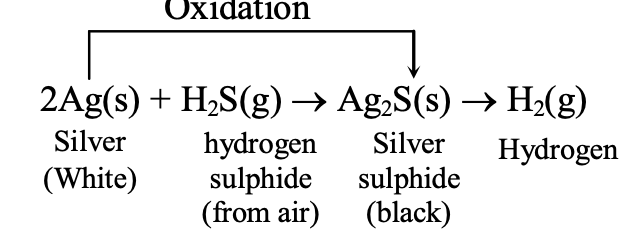
This reaction is called the corrosion of silver.
(ii) Rancidity: Oxidation has damaging effects on food and eatables. When fats and oils present in butter and margarine are oxidised, they become rancid. As a result, their smell and taste change. They become quite unpleasant. This is known as rancidity. It can be checked in a number of ways.
(a) Manufacturer sometimes adds certain food additives to the food materials. These are known as antioxidant and check their oxidation.
(b) Keeping food in airtight containers prevents its oxidation.
(c) Refrigeration of food also slows down rancidity because the temperature inside refrigerator is very low and direct contact with air or oxygen is avoided.
(d) Chips manufacturers generally flush their bags with nitrogen before packing so that they may not be oxidised.
Prevention from Rancidity :
- Adding antioxidants (Reducing agents) like BHA (Butylated Hydroxy Anisole) and BHT (Butylated Hydroxy-Toluene) to foods containing fats and oils.
- Packaging fat and oil containing foods in nitrogen gas (inert gas).
- Keeping food in a refrigerator.
- Storing food in air tight containers.
- Storing foods away from light.
TYPES OF CHEMICAL REACTIONS BASED ON HEAT CHANGES
Depending upon the kind of heat change during a reaction, the chemical reactions are classified into two types :
- Exothermic Reactions
- Endothermic Reactions
EXOTHERMIC REACTIONS:
The term exothermic is taken from Greek word exotherm (exo-out, therm-heat) which means heat goes out. Thus a reaction in which heat is released or produced is called an exothermic reaction.
Some examples of exothermic reactions:
(a) When nitrogen and hydrogen combine then, ammonia (NH3) is formed and heat is liberated. Thus, formation of ammonia is an exothermic reaction.
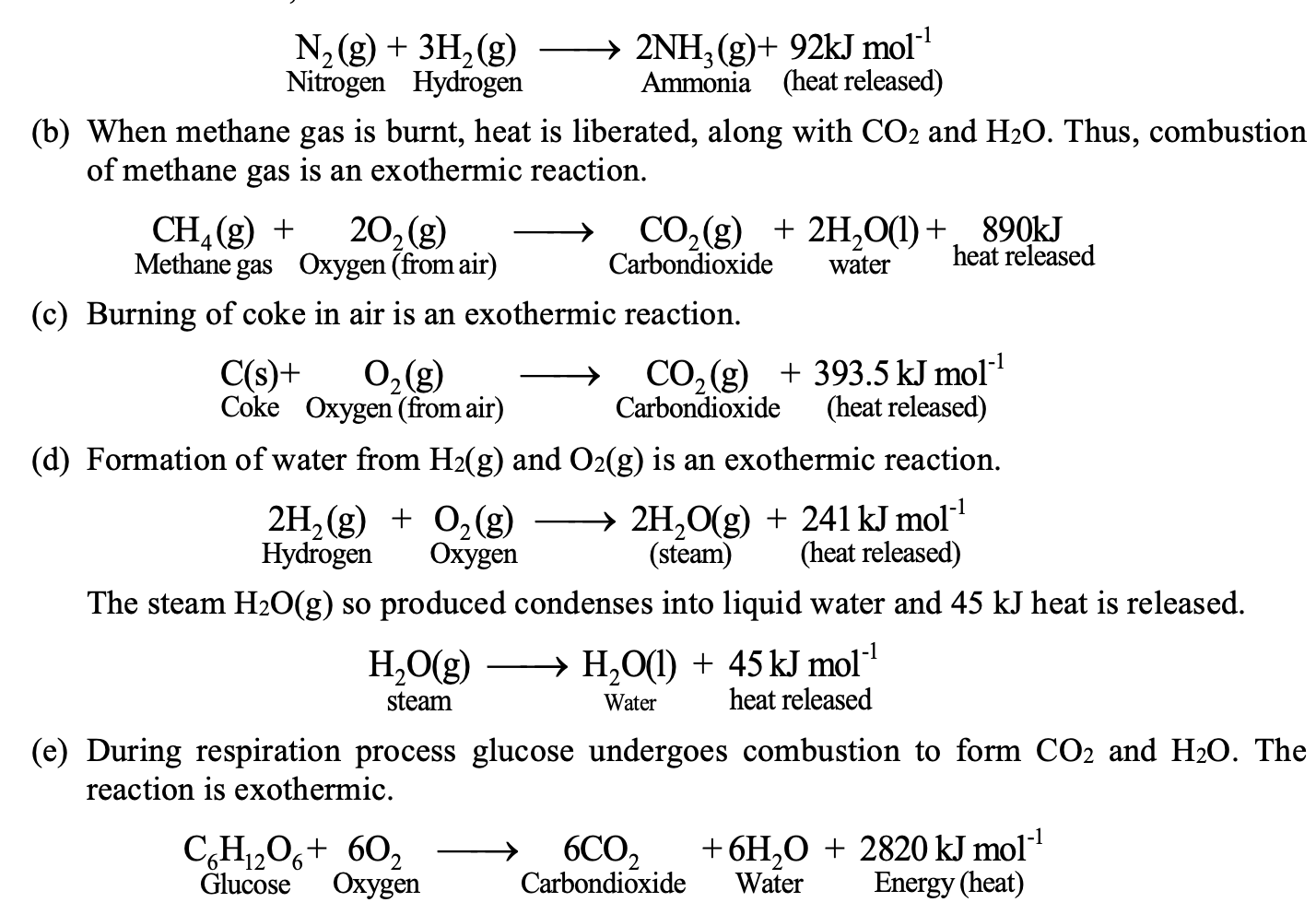
Why and when a chemical reaction is exothermic?
We know that in a chemical reaction, heat is supplied to break the bonds in the reactants and heat is released due to bond formation in the products. When the heat released due to bond formation in products is greater than the heat supplied to break the bonds in reactants, then the reaction is exothermic.
For example formation of H2O from (H2) and (O2) is exothermic because the energy released in the formation of two covalent bonds in H2O is more than the energy, required to break
H – H bonds in H2 and O = O bonds in O2.
ENDOTHERMIC REACTIONS:
The term endothermic is taken from the Greek word endotherm (endo-in, therm-heat) which means heat is taken in. Thus, a reaction in which heat is absorbed is called the endothermic reaction.
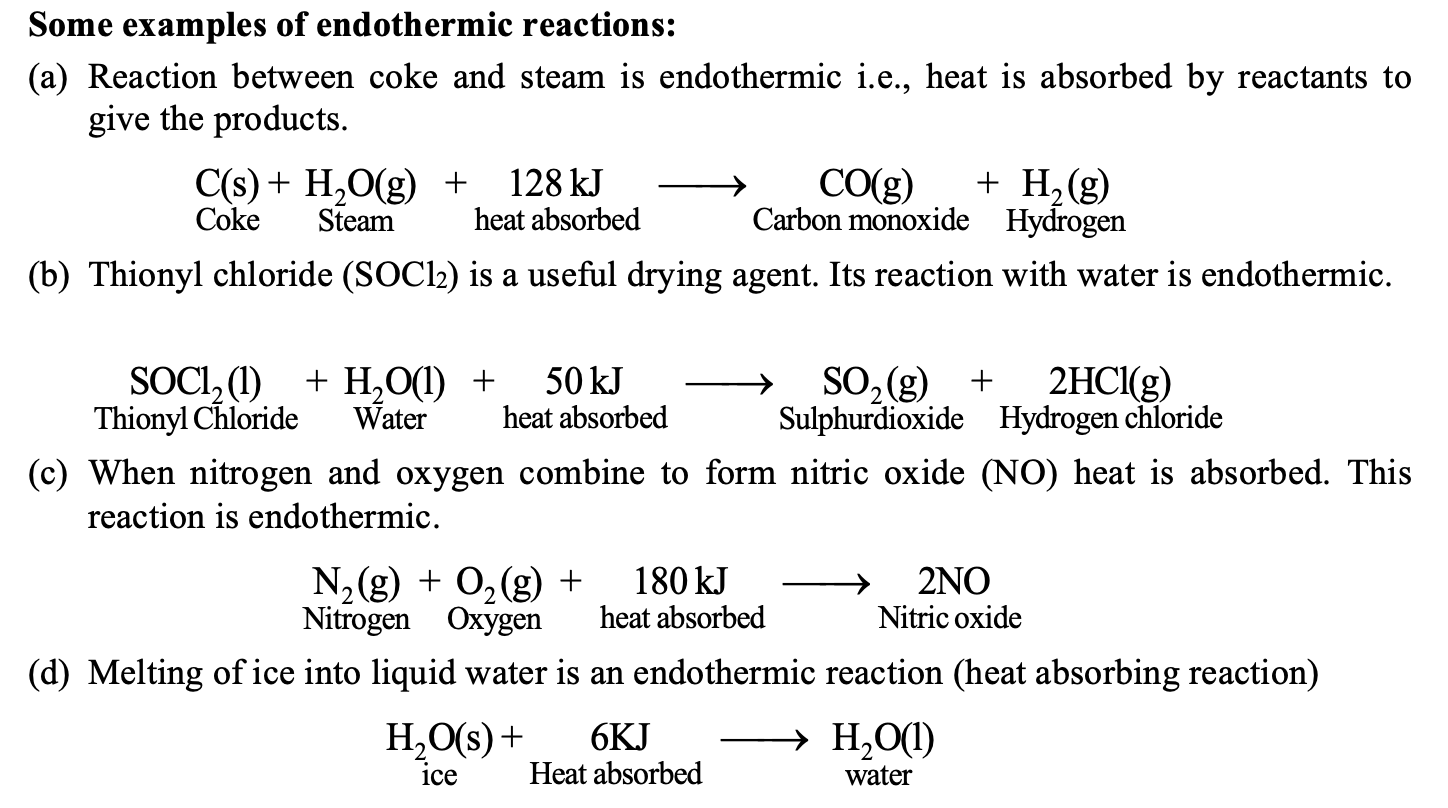
Why and when a chemical reaction is endothermic?
In a chemical reaction when heat required to break the bonds in all reactants is greater than the heat released in the bond formation of the products, then the reaction is said to be endothermic.
For example: Formation of nitric oxide from N2 and O2 is endothermic because the heat required to break N – N bonds in N2 and (O=O) bonds in O2 is more than the heat released in the formation of two covalent bonds in NO.
Ex.: Define a chemical equation?
Sol. The short-hand method of representing a chemical reaction in terms of symbols and formulae of the different reactants and products is called a chemical equation.
Ex.: Why should a magnesium ribbon be cleaned before burning in air?
Sol. A magnesium ribbon is cleaned by rubbing with a paper to remove the protective layer of basic magnesium carbonate so that it readily combines with air.
Ex.: List the effects of oxidation in our daily life. Are these effects useful or harmful? Justify.
Sol. The most common effects of oxidation in everyday life are:
(a) Corrosion of metals
(b) Rancidity
Both the effects are harmful, because corrosion eats away and spoils the metals and rancidity spoils the flavour and taste of oily food. But inside our body, oxidation of food provides us energy. This process is known as respiration. Therefore oxidation reactions are not always harmful.