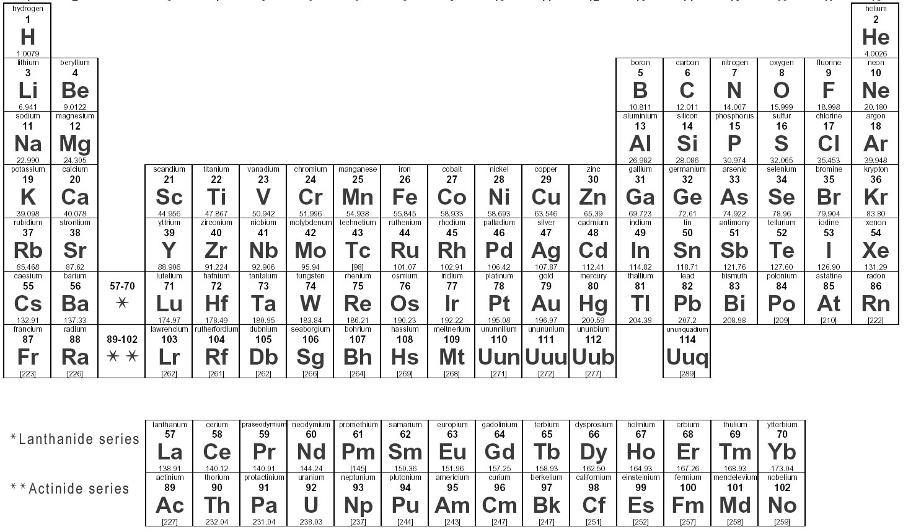
The Periodic Table: A Comprehensive Guide to Element Classification
What is the Periodic Table?
The periodic table is a systematic arrangement of all known chemical elements organized according to their properties. This fundamental tool of chemistry places elements with similar characteristics in the same vertical columns (groups) while separating dissimilar elements into different positions. Currently, 114 elements have been identified, making the periodic table an indispensable reference for understanding chemical behavior and relationships.
The need for such classification became apparent as the number of known elements grew from approximately 30 in 1800 to over one hundred today. Studying each element individually would be impractical, prompting scientists to develop organizational systems that reveal patterns and predict properties.
Early Attempts at Element Classification
Dobereiner's Triads (1817)
German chemist J.W. Dobereiner made one of the first systematic attempts to classify elements by organizing them into groups of three, called triads. His key observation was that the atomic mass of the middle element was approximately the arithmetic mean of the other two elements in the triad. For example, in the triad of lithium (Li, atomic mass 7), sodium (Na, atomic mass 23), and potassium (K, atomic mass 39), sodium's atomic mass (23) equals the average of lithium and potassium: (7 + 39) ÷ 2 = 23.
Other notable triads included:
- Sulfur, Selenium, Tellurium (32, 79, 128)
- Chlorine, Bromine, Iodine (35.5, 80, 127)
- Calcium, Strontium, Barium (40, 88, 137)
However, this classification system had significant limitations. It applied only to a small number of elements and failed to account for elements with nearly identical atomic masses, such as iron, cobalt, and nickel. Consequently, Dobereiner's approach was eventually discarded.
Newlands' Law of Octaves (1866)
Building on earlier work, English chemist John Newlands arranged elements in order of increasing atomic mass, starting with hydrogen (atomic mass 1) and extending to thorium (the 56th element). He discovered that every eighth element exhibited properties similar to the first, reminiscent of the eight notes in a musical octave. This pattern led him to propose the "Law of Octaves."
For instance, lithium was the first element in his arrangement, and sodium the eighth element from lithium displayed remarkably similar properties. However, Newlands' system suffered from critical flaws:
- The pattern only worked for elements up to calcium (Ca)
- It assumed only 56 elements existed in nature, with no provision for future discoveries
- To force elements into his table, Newlands placed dissimilar elements (such as cobalt, nickel, fluorine, chlorine, and bromine) in the same column, despite their vastly different properties
- Iron was separated from cobalt and nickel, despite having similar properties
Mendeleev's Breakthrough: The First Comprehensive Periodic Table
Development and Fundamental Principles
Russian chemist Dmitri Mendeleev revolutionized element classification in 1869 by publishing the first comprehensive periodic table based on atomic mass. When he began his work, only 63 elements were known. Mendeleev carefully examined relationships between atomic mass and both chemical and physical properties, focusing particularly on compounds formed with hydrogen and oxygen.
His innovative approach involved writing each element's properties on separate cards, including formulas for its hydrides and oxides. By arranging cards with similar properties in vertical columns, he noticed that elements naturally organized themselves in order of increasing atomic mass, with similar properties recurring at regular intervals.
This observation led to Mendeleev's Periodic Law: "The physical and chemical properties of elements are a periodic function of their atomic masses." This concept of periodicity the repetition of properties at regular intervals became the foundation of modern chemistry.
Structure of Mendeleev's Periodic Table
Mendeleev's table consisted of:
- Eight vertical columns (groups): Designated I through VIII, with groups I-VII subdivided into subgroups A and B
- Seven horizontal rows (periods): Numbered 1 through 7
- Group A elements: Normal or representative elements
- Group B elements: Transition elements
- Group VIII: Nine transition elements arranged in three triads, appearing in the 4th, 5th, and 6th periods
Revolutionary Achievements
Mendeleev's periodic table demonstrated remarkable foresight:
- Reversed atomic mass order when necessary: He prioritized similar properties over strict atomic mass sequence, placing cobalt (58.9 u) before nickel (58.71 u) and tellurium (127.6 u) before iodine (126.9 u)
- Left strategic gaps for undiscovered elements: Mendeleev predicted the existence and properties of missing elements using the prefix "Eka" (Sanskrit for "one"). His predictions proved remarkably accurate:
| Property | Eka-aluminium (Predicted) | Gallium (Found) | Eka-silicon (Predicted) | Germanium (Found) |
|---|---|---|---|---|
| Atomic mass | 68 | 69.7 | 72 | 72.6 |
| Density (g/cm³) | 5.9 | 5.94 | 5.5 | 5.36 |
| Melting point | Low | 303.2 K | High | 1231 K |
| Oxide formula | R₂O₃ | Ga₂O₃ | RO₂ | GeO₂ |
| Chloride formula | RCl₃ | GaCl₃ | RCl₄ | GeCl₄ |
- Accommodated noble gases: When noble gases were discovered in 1900, they were seamlessly added as a zero group without disrupting the existing organization
Limitations of Mendeleev's Approach
Despite its success, Mendeleev's table had several shortcomings:
- Uncertain position of hydrogen: Could be placed with alkali metals or halogens
- Isotope problem: Isotopes with different atomic masses should theoretically occupy different positions, yet they share identical chemical properties
- Anomalous pairs: Several element pairs violated the increasing atomic mass order (Ar-K, Co-Ni, Te-I)
- Misplaced elements: Copper, silver, and gold were grouped with alkali metals despite significant property differences
- No explanation for periodicity: Mendeleev could not explain why properties repeated at regular intervals
The Modern Periodic Table: Based on Atomic Number
Moseley's Discovery and the Modern Periodic Law
In 1913, English physicist Henry Moseley demonstrated that atomic number not atomic mass determines an element's properties. The atomic number represents the number of protons in the nucleus and increases by exactly one from element to element. This insight led to the Modern Periodic Law: "Physical and chemical properties of an element are a periodic function of its atomic number."
Cause of Periodicity Explained
The modern periodic table reveals that periodicity results from the recurrence of similar valence shell electron configurations. Elements in the same group share the same number of valence electrons, which determines their chemical behavior. For example, all alkali metals have one electron in their outermost shell (ns¹):
| Element | Atomic Number | Electronic Configuration |
|---|---|---|
| Lithium (Li) | 3 | 2, 1 |
| Sodium (Na) | 11 | 2, 8, 1 |
| Potassium (K) | 19 | 2, 8, 8, 1 |
| Rubidium (Rb) | 37 | 2, 8, 18, 8, 1 |
| Caesium (Cs) | 55 | 2, 8, 18, 18, 8, 1 |
| Francium (Fr) | 87 | 2, 8, 18, 32, 18, 8, 1 |
Structure of the Long Form Periodic Table
The long form periodic table (also called Bohr's table) organizes elements based on their electronic configuration:
Periods (Horizontal Rows)
| Period | Number of Elements | Classification | Valence Shell |
|---|---|---|---|
| 1st (n=1) | 2 | Very short period | K shell |
| 2nd (n=2) | 8 | Short period | L shell |
| 3rd (n=3) | 8 | Short period | M shell |
| 4th (n=4) | 18 | Long period | N shell |
| 5th (n=5) | 18 | Long period | O shell |
| 6th (n=6) | 32 | Very long period | P shell |
| 7th (n=7) | 25 | Incomplete period | Q shell |
Each period begins with an alkali metal (ns¹ configuration) and ends with a noble gas (ns²np⁶ configuration, except helium with 1s²). Elements within a period have different valence electron numbers, resulting in diverse properties.
Groups (Vertical Columns)
The modern table contains 18 groups numbered 1 through 18:
- Groups 1 & 2: Alkali metals and alkaline earth metals
- Groups 13-17: Representative elements
- Group 17: Halogens
- Group 18: Noble gases (zero group, with valency of zero)
- Groups 3-12: Transition elements
Elements within the same group share similar valence shell configurations and therefore exhibit similar chemical properties.
Formulas and Relationships in Periodic Classification
| Concept | Formula/Relationship | Explanation |
|---|---|---|
| Dobereiner's Triad | Atomic mass of middle element = (Mass of 1st + Mass of 3rd) ÷ 2 | Middle element's mass is the arithmetic mean |
| Newlands' Octaves | Property of 8th element ≈ Property of 1st element | Every eighth element shows similar properties |
| Mendeleev's Periodic Law | Properties = f(Atomic Mass) | Properties are periodic functions of atomic mass |
| Modern Periodic Law | Properties = f(Atomic Number) | Properties are periodic functions of atomic number |
| Valence Electron Configuration | Group 1: ns¹, Group 2: ns², Group 17: ns²np⁵, Group 18: ns²np⁶ | Similar outer shell configurations explain similar properties |
| Period Number | Period number = Number of electron shells | Determines which valence shell is being filled |
Advantages of the Modern Periodic Table
The atomic number-based organization offers significant improvements:
- Fundamental basis: Atomic number is more fundamental than atomic mass
- Resolves isotope problem: Isotopes share the same atomic number and thus occupy the same position
- Separates isobars: Elements with identical mass but different atomic numbers (like Ar-40 and Ca-40) are correctly placed in different positions
- Explains periodicity: Similar valence electron configurations recur at regular intervals
- Easy to memorize and reproduce: Logical organization aids learning
Remaining Limitations
Even the modern table has minor limitations:
- Hydrogen's position remains somewhat ambiguous (could fit with Group 1 or Group 17)
- Inner transition elements (lanthanoids and actinoids) are placed separately below the main table for spatial convenience
Need for Classification
We have studied earlier that matter around us is present in the form of elements compounds and mixtures and the elements contain only one type of atoms. Around the year 1800 only 30 elements were known. Till date 114 elements are known.
It is very difficult to study the chemistry of more than one hundred elements individually. This practical problem was felt by the scientists and after numerous attempts the scientists were ultimately successful in arranging the elements. This arrangement of elements provided a proper classification of elements leading to the formation of periodic table.
Early attempts to classify elements:
(a) Metals and Non-Metals:
Among the earlier classifications, Lavoisier classified the elements as metals and non-metals. However, this classification proved to be inadequate. In 1803, John Dalton published a table of relative atomic weights (now called atomic masses). This formed an important basis of classification of elements.
(b) Dobereiner's Triads:
(i) In 1817, J.W. Dobereiner a German Chemist gave this arrangement of elements.
(a) He arranged elements with similar properties in the groups of three called triads.
(b) According to Dobereiner the atomic mass of the central element was merely the arithmetic mean of atomic masses of the other two elements.
e.g.
Atomic mass of sodium = Atomic mass of lithium + Atomic mass of postassium / 2 = 7 + 39 / 2 = 23
Some examples of triads are given in the table:
| S.No. | Triads | Relative atomic masses | Average of atomic masses of the 1st and the 3rd element |
| 1. | S, Se, Te | 32, 79, 128 | (32 + 128)/2 = 80 |
| 2. | Cl, Br, I | 35.5, 80, 127 | (35.5 + 127)/2 = 81.25 |
| 3. | Ca, Sr, Ba | 40, 88, 137 | (40 + 137)/2 = 88.5 |
(ii) Limitations of Dobereiner's Classification:
(a) Atomic mass of the three elements of some triads is almost same.
e.g. Fe, Co, Ni and Ru, Rh, Pd
(b) It was restricted to few elements, therefore discarded.
NEWLANDS’ LAW OF OCTAVES
Dobereiner’s attempt encouraged other scientists to correlate the atomic masses of the elements with their properties.
In 1866, Newlands arranged the elements in order of increasing atomic masses. He started with hydrogen having atomic mass 1 and ended at thorium which was the 56th element. He found that the properties of every eighth element were similar to the first, like 8th note of a musical scale. Therefore, he called it as “Newlands’ law of octaves”.
Newlands’s Octaves
| sa
(do) |
re
(re) |
ga(mi) | ma(fa) | pa(so) | da(la) | ni(ti) |
| H | Li | Be | B | C | N | O |
| F | Na | Mg | Al | Si | P | S |
| Cl | K | Ca | Cr | Ti | Mn | Fe |
| Co and Ni | Cu | Zn | Y | In | As | Se |
| Br | Rb | Sr | Ce and La | Zr | - | - |
In Newlands’ octaves properties of lithium and sodium were similar and sodium is the eighth element from Lithium.
Limitations:
(i) It was found that the law of octaves was applicable only upto Ca or we can say that it is applicable only for the lighter elements.
After Ca every eighth element did not possess properties similar to that of first one.
(ii) It was assumed by Newland that only 56 elements existed in nature and no more elements would be discovered in the future. But later on many more elements were discovered whose properties did not fit into the law of octaves.
(iii) In order to fit elements into his table, Newlands not only adjusted two elements into the same slot, but also put some unlike elements under the same slot.
For Example, Co and Ni are in the same slot and these are placed in the same column as Fluorine, Chlorine and Bromine which have very different properties than these elements while iron which have properties similar to that of Cobalt and Nickel has been placed in a different column.
Making Order out of Chaos-Mendleev's Periodic Table
While Dobereiner initiated the study of periodic relationship, it was Mendeleev who was responsible for publishing the periodic law for the first time. The first breakthrough in the classification of elements was provided by Mendeleev. He was regarded as the main contributor to the early development of the periodic table. In his periodic table elements were arranged on the basis of their fundamental properties i.e. atomic mass.
A periodic table may be defined as an arrangement which classifies all the known elements on the basis of their properties in such a way that similar elements are placed in the same vertical column while dissimilar elements are placed in different columns.
When he started his work on classification of elements, only 63 elements were known. He carefully examined the relationship between the atomic mass of the element and their chemical and physical properties. Among the chemical properties he concentrated on the compounds formed by the elements with hydrogen and oxygen as they are very reactive and formed compound with most of the elements. Therefore, the formula of oxides and hydrides formed by the elements were taken as the basis of classification of elements.
Mendeleev took 63 cards, each card representing an element, where he wrote down the properties of that element as well as the formulae of its hydride and oxide. He then separated and arranged the elements with similar properties and pinned the cards on the wall one after the other in a vertical column. When he observed these cards after arranging he found that most of the elements automatically got arranged in order of their increasing atomic masses. It was also observed that elements with similar properties occur after a certain interval or In other words there was recurrence of elements with similar physical and chemical properties after a regular interval. On this basis, he formulated a periodic law which states that
“the physical and chemical properties of elements are a periodic function of their atomic masses”.
Periodic function means that the properties of the elements get repeated after certain regular intervals. His law can also be stated as
“when elements are arranged in order of their increasing atomic masses, elements with similar properties are repeated after a certain regular intervals”.
This repetition of properties is called periodicity of properties.
Mendeleev's Periodic Table
Mendeleev arranged the known 63 elements in increasing order of their atomic masses in horizontal rows called periods in such a way that elements with similar properties fall under the same vertical column called groups.
Mendeleev's Periodic Table
| Group | I | II | III | IV | V | VI | VII | VIII |
| Oxide Hydride |
R₂O RH |
RO RH₂ |
R₂O₃ RH₃ |
RO₂ RH₄ |
R₂O₅ RH₃ |
RO₃ RH₂ |
R₂O₇ RH |
RO₄ |
|
Periods ↓ |
A B | A B | A B | A B | A B | A B | A B | Transition series |
| 1 |
H 1.008 |
|||||||
| 2. |
Li 6.939 |
Be 9.012 |
B 10.81 |
C 12.011 |
N 14.007 |
O 15.999 |
F 18.998 |
|
| 3. |
Na 22.99 |
Mg 24.31 |
Al 29.98 |
Si 28.09 |
P 30.974 |
S 32.06 |
Cl 35.453 |
|
|
4. First series: second series: |
K 39.102 Cu 63.54 |
Ca 40.08 Zn 65.37 |
Sc 44.96 Ga 69.72 |
Ti 47.90 Ge 72.59 |
V 50.94 As 74.92 |
Cr 50.20 Se 78.96 |
Mn 54.94 Br 79.909 |
Fe 55.85 Co 58.93 Ni 58.71 |
|
5.First series: second series: |
Rb 85.47 Ag 107.87 |
Sr 87.62 Cd 112.40 |
Y 88.91 In 114.82 |
Zr 91.22 118.69 |
Nb 92.91 Sb 121.75 |
Mo 95.94 Te 127.60 |
Tc 99 I 126.90 |
Ru 101.07 Rh Pd 106.4 |
|
6.First series: second series: |
Cs 132.90 Au 196.97 |
Ba 137.34 Hg 200.59 |
La 138.91 Tl 204.37 |
Hf 178.49 Pb 207.19 |
Ta 180.95 Bi 208.98 |
W 183.85 |
Os 190.2 Ir 192.2 Pt 195.09 |
CHARACTERISTIC FEATURES OF MENDELEEV’S PERIODIC TABLE:
Mendeleev’s periodic table consists of
Eight vertical columns: Groups
They are designated as I, II, III, IV, V, VI, VII, and VIII. Except group VIII, each group is further sub-divided into two sub groups namely A and B. The elements which lie on the left hand side of the column constitute sub group A while the elements on the right hand side of that column constitute sub-group B.
Elements of sub-group A elements are known as normal or representative elements while sub group B elements are known as Transition elements. This sub division is made due to difference in their properties.
| Normal/Representative element | Transition elements |
| I A | I B |
| II A | II B |
| III A | III B |
| IV A | IV B |
| V A | V B |
| VI A | VI B |
| VII A | VII B |
Group VIII contain 9 transition elements arranged in a group of 3 elements and these lie in the 4th, 5th and 6th period.
Seven horizontal rows: Periods
In Mendeleev’s periodic table there were 7 horizontal columns present which we call as periods and are numbered from 1 to 7.
Nobel gases were not known at the time of Mendeleev. So there was no group for these gases but in 1900 when Nobel gases were discovered new group called zero group was introduced.
Achievements of Mendeleev's Periodic Table:
Mendeleev’s Periodic table was based mainly on two things, which are
(i) Increasing atomic mass.
(ii) Grouping of similar elements together.
In order to place the elements with similar properties together in some cases Mendeleev had to place an element with a slightly greater atomic mass before an element which has slightly lower atomic mass.
Ex. Cobalt (atomic mass 58.9 u) appeared before Nickel (atomic mass = 58.71 u) and Tellurium (atomic mass 127.6 u) was placed before Iodine (126.9 u).
Mendeleev also left some gaps in his periodic table for those elements which were not known or discovered at that time. He was sure about the existence of those elements and also predicted the properties of these elements. Later on, when these elements were discovered their properties were found to be very close to that which were predicted by him.
Ex. Scandium, Gallium and Germanium discovered later have properties similar to Eka-boron, Eka-aluminium and Eka-silicon.
‘Eka’ word was used as a prefix by Mendeleev for the name of the succeeding element in the same group which were not discovered. Therefore, the element which would fill the gap after boron was called eka-boron.
|
PROPERTY |
Eka-aluminium (R) (predicted) |
Gallium (Found) |
Eka-silicon (R) (predicted) |
Germanium (found) |
|
Atomic mass Density (g cm-3) Melting point (K) Formula of Oxide Formula of Chloride |
68 5·9 Low R2O3 RCl3 |
69·7 5·94 303·2 Ga2O3 GaCl3 |
72 5·5 High RO2 RCl4 |
72·6 5·36 1231 GeO2 GeCl4 |
The above data proved the correctness and usefulness of the Mendeleev’s periodic table. After the discovery of noble gases in 1900 he placed these gases in a separate group called zero group without disturbing the existing order as these gases have very different properties than other elements.
Limitations of Mendeleev’s Periodic Table:
Inspite of many advantages, the Mendeleev’s periodic table has certain defects also. Some of these are given below.
(a) Position of Hydrogen:
Position of Hydrogen in the periodic table is uncertain. It has been placed in 1A group with alkali metals, but certain properties of Hydrogen resemble those of halogens. So, it may be placed in the group of halogens as well.
(b) Position of Isotopes:
Isotopes are the atoms of the same element having different atomic masses. Therefore, According to Mendeleev’s classification these should be placed at different places depending upon their atomic masses. For example, hydrogen isotopes with atomic masses 1, 2 and 3 should be placed at three places. However, isotopes have not been given separate places in the periodic table because of their similar properties.
(c) Anomalous pairs of Elements:
In certain pairs of elements, the increasing order of atomic masses was not obeyed. In these, Mendeleev placed elements according to similarities in their properties and not in increasing order of their atomic masses.
For example,
- The atomic mass of argon is 39.9 and that of Potassium 39.1. But Argon is placed before Potassium in the periodic table.
- The positions of Cobalt and nickel are not in proper order. Cobalt (at. mass = 58.9) is placed before Nickel (at. mass = 58.7).
- Tellurium (at. mass = 127.6) is placed before Iodine (at. Mass = 126.9).
(d) Some similar elements are separated, in the periodic table. For example Copper (Cu) and Mercury (Hg), Barium (Ba) and Lead (Pb). On the other hand, some dissimilar elements have been placed together in the same group.
e.g., Copper (Cu), Silver (Ag) and Gold (Au) have been placed in group I, along with alkali metals. Similarly, Manganese (Mn) is placed in the group of halogens.
(e) Cause of periodicity:
Mendeleev could not explain the cause of periodicity among the elements.
Modern Periodic Table
In 1913, an English physicist, Henry Moseley showed that the physical and chemical properties of the atoms of the elements are determined by their atomic number and not by their atomic masses. Consequently, the periodic law was modified.
Modern Periodic Law (Moseley's Periodic Law):
“Physical and chemical properties of an element are the periodic function of its atomic number”. The atomic number gives us the number of protons in the nucleus of an atom and this number increases by one in going from one element to the next. Elements, when arranged in the order of increasing atomic number Z, lead us to the classification known as the Modern Periodic Table. Prediction of properties of elements could be made with more precision when elements were arranged on the basis of increasing atomic number.
PERIODICITY:
The repetition of elements with similar properties after certain regular intervals, when the elements are arranged in order of increasing atomic number, is called periodicity.
Cause of Periodicity:
The periodic repetition of the properties of the elements is due to the recurrence of similar valence shell (outermost shell) electronic configuration after certain regular intervals.
e.g., Alkali metals have similar electronic configuration (ns1) and therefore have similar properties.
Alkali Metals:
|
At. N. |
Element |
Symbol |
Electronic Configuration |
|
3 |
Lithium |
Li |
2,1 |
|
11 |
Sodium |
Na |
2,8,1 |
|
19 |
Potassium |
K |
2,8,8,1 |
|
37 |
Rubidium |
Rb |
2,8,18,8,1 |
|
55 |
Caesium |
Cs |
2,8,18,18,8,1 |
|
87 |
Francium |
Fr |
2,8,18,32,18,8,1 |
Long form of Periodic Table:
(i) The long form of periodic table is based upon Modern periodic law. Long form of periodic table is the contribution of Range, Werner, Bohr and Bury.
(ii) This table is also referred to as Bohr's table since it follows Bohr's scheme of the arrangement of elements into four types based on electronic configuration of elements.
(iii) Long form of periodic table consists of horizontal rows (periods) and vertical columns (groups).
Description of Periods:
(i) A horizontal row of a periodic table is called a period.
(ii) There are seven periods numbered as 1, 2, 3, 4, 5, 6 and 7.
(iii) Each period consists of a series of elements having the same valence shell.
(iv) Each period starts with an alkali metal having outermost shell electronic configuration ns1.
(v) Each period ends with a noble gas with outermost shell electronic configuration ns2, np6 except Helium having outermost electronic configuration 1s2.
(vi) Each period starts with the filling of a new energy level.
(a) 1st Period:
This period is called very short period because this period contains only 2 elements 1H and 2He.
(b) 2nd and 3rd Periods:
These periods are called short periods because these periods contain 8 elements. 2nd period starts from 3Li to 10Ne and 3rd period starts from 11Na to 18Ar.
(c) 4th and 5th Periods:
These periods are called long periods because these periods contain 18 elements. 4th period starts from 19K to 36Kr and 5th period starts from 37Rb to 54Xe.
(d) 6th Period:
This period is called very long period. This period contains 32 elements. Out of the 32 elements14 elements belong to Lanthanoid Series (58Ce to 71Lu). 6th period starts from 55Cs to 86Rn.
(e) 7th Period:
This period is called as incomplete period. It contains 25 elements. Out of the 25 elements 14 elements belong to Actinoid Series (90Th to 103Lr). 7th period starts from 87Fr to 111Rg.
|
Periods |
No. of Elements |
Called as |
|
(1st) n = 1 |
2 |
Very short period |
|
(2nd) n = 2 |
8 |
Short period |
|
(3rd) n = 3 |
8 |
Short period |
|
(4th) n = 4 |
18 |
Long period |
|
(5th) n = 5 |
18 |
Long period |
|
(6th) n = 6 |
32 |
Very long period |
|
(7th) n = 7 |
25 |
Incomplete period |
Different elements belonging to a particular period have different electronic configurations and have different number of valence electrons. That is why elements belonging to a particular period have different properties.
Description of Groups:
(i) A vertical column of elements in the periodic table is called a group.
(ii) There are eighteen groups numbered as 1, 2, 3, 4, 5 ……………… 13, 14, 15, 16, 17, 18.
(iii) A group consists of a series of elements having similar valence shell electronic configuration and hence exhibit similar properties.
e.g. Li, Na, K belong to the same group and have 1 electron in their valence shell.
(iv) The group 18 is also known as zero group because the valency of the elements of this group is zero. The elements of 18thor zero group are called noble gases.
(v) The elements present in groups 1, 2, 13 to 17 are called normal or representative elements.
(vi) Elements of group 1 and 2 are called alkali metals and alkaline earth metals respectively.
(vii) Elements present in group 17 are called halogens.
Merits of long form of periodic table:
(i) The long form of periodic table is based on atomic number. Atomic number is a more fundamental property of an element as compared to atomic mass.
(ii) In the long form of periodic table, different isotopes can be placed at the same place because they have same atomic number. On the other hand, isobars such as Ar (40) and Ca (40) have to be placed at different places due to their different atomic numbers.
(iii) The long form of periodic table can explain why all the elements in a group have similar properties while the elements in a period have different properties:-
The basis for periodicity of elements is the similar electronic configuration of the outermost shell of elements of the same group. The similar electronic configuration of the elements is repeated at regular intervals so the properties of the elements are also repeated at regular intervals.
(iv) It is easy to remember and reproduce the table.
LIMITATIONS OF LONG FORM OF PERIODIC TABLE:
(i) Position of hydrogen is not accurate.
(ii) Inner transition elements (Lanthanoids and Actinoids) have been given separate positions below in the periodic table.
Periodicity in Properties:
(i) The electronic configurations of the atoms display periodic variations with increase in atomic number.
(ii) The elements exhibit periodic variations of physical and chemical properties. Following are some of the important properties of the elements:
(a) Valency (b) Atomic size (c) Metallic and non - metallic character
Valency:
We know that valency of an element is determined by the number of electrons present in outermost shell or valence shell. Valency of an element is also determined by the number of electrons an element loses or gains while combining with atoms of other elements in order to become stable or in other words the combining capacity of an element is called its valency.
Variation of valency in a period:
On moving from left to right in a period the valency first increases from 1 – 4 and than decreases to zero.
In a period, the valency an element is either equal to the number of electrons in the valence shell or eight minus the number of electrons in the valence shell. This can be better understood by the following table.
For the Elements in the third period:
| Group No. | 1 | 2 | 13 | 14 | 15 | 16 | 17 | 18 |
| Atomic No. | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 |
| Symbol | Na | Mg | Al | Si | P | S | Cl | Ar |
| Electronic configuration | 2, 8, 1 | 2, 8, 2 | 2, 8, 3 | 2, 8, 4 | 2, 8, 5 | 2, 8, 6 | 2, 8, 7 | 2, 8, 8 |
| No. of valence
electrons |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Valency | 1 | 2 | 3 | 4 | 3 | 2 | 1 | 0 |
It is clear from the above table that Na, Mg and Al has 1, 2, and 3 valence electrons respectively therefore these elements can easily lose electrons to acquire the stable electronic configuration of the nearest noble gas i.e.,
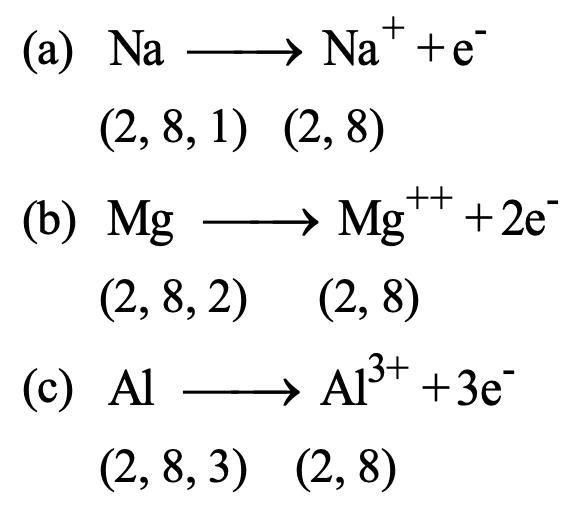
Silicon has 4 electrons in the valence shell therefore, it can achieve the stable configuration either by losing or gaining or sharing 4 electrons.
As a lot of energy is required to either lose or gain 4 electrons, therefore silicon attains the stable configuration of the nearest noble gas by sharing 4 electrons. Thus, Si has the valency of 4.
Similarly P,S, and Cl requires 3, 2, & 1 electrons respectively to complete their octet, so their valency is 3, 2 and 1 respectively.
The last element is Ar which has 8 electrons in its outermost orbit/shell so it doesn’t require to gain, lose or share electrons in order to complete its octet. Therefore, it does not have any tendency to form bonds. Thus, the valency of Ar is zero.
Variation of valency in a group:
As we know that all the elements in a group have similar outer electronic configuration i.e. they have same number of valence electrons. Therefore, the valency of all the elements in a group is fixed or same. For example, elements of group 1, 2, 3 have valency 1, 2, 3 respectively. Similarly group 17 requires 1 electron to complete its octet so its valency is also 1.
Atomic Size:
The term atomic size refers to the radius of an atom. In general atomic size may be considered as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
Variation of atomic size in a period:
Within each period, the atomic radii decrease with increase in atomic number. e.g., Atomic radii decrease from Lithium to Fluorine in the second period.
Reason:
The decrease of atomic radii along a period can be explained on the basis of increase in nuclear charge. On moving from left to right across the period, the nuclear charge increases progressively by one unit but the additional electron goes to the same shell. As a result the electrons are pulled closer to the nucleus by the increased nuclear charge. This causes a decrease in atomic size.
Atomic Radii of Elements of Second Period:
| Element | 3Li | 4Be | 5B | 6C | 7N | 8O | 9F | 10Ne |
| Nuclear Charge | +3 | +4 | +5 | +6 | +7 | +8 | +9 | +10 |
| Configuration | 2s1 | 2s2 | 2s2 2p1 | 2s2 2p2 | 2s2 2p3 | 2s2 2p4 | 2s2 2p5 | 2s2 2p6 |
| Atomic Radii (pm) | 133 | 111 | 88 | 77 | 75 | 74 | 72 | 160 |
The values given in the table show abrupt increase in the atomic size of Ne. This is due to the reason that the values given for other elements are covalent radii whereas that for Ne it is Vander Waals radius as it does not form covalent bond due to its stable configuration.
Variation of atomic radii within a group:
The atomic radii increase from top to bottom within a group of the periodic table.
Atomic Radii of Alkali Metals and Halogens:
|
Alkali Metals |
Halogens |
||
| Element | Atomic Radius (pm) | Element (pm) | Atomic Radius (pm) |
| Li
Na K Rb Cs |
133
157 201 216 235 |
F
CI Br I |
64
99 114 133 |
Reason:
In moving down a group, the nuclear charge increases with increase in atomic number, but at the same time there is a progressive increase in the number of energy levels. Since, the effect of additional energy level is more pronounced than the effect of increased nuclear charge, the distance of the outermost electron from the nucleus increases on going down the group.
- Atomic radii increase down the group.
- Atomic radii decrease across the period.
Metallic and Non-Metallic Character:
Metals:
The metals are characterised by their nature of readily giving up the electrons.
(i) Metals comprise of more than 75% of all known elements and most of them appear on the left hand side of the periodic table.
(ii) Metals are usually solid at room temperature (except Mercury).
(iii) They have high melting and boiling points and are good conductors of heat and electricity.
Non–Metals:
(i) Non-metals do not lose electrons but take up electrons to form corresponding anions.
(ii) Non-metals are located at the top right hand side of the periodic table.
(iii) Non-metals are usually solids or gases (except Bromine which is liquid) at room temperature with low melting and boiling points.
(iv) They are poor conductors of heat and electricity.
Metalloids (Semimetals):
(i) Some elements lying at the border of metallic and non-metallic behaviour possess the properties that are characteristics of both metals and non - metals. These elements are called semimetals or metalloids.
(ii) The metalloids comprise of the elements B, Si, Ge, As, Sb, Te and Po.
(iii) Variation of metallic character across a period: Metallic character decreases along a period due to increase in ionisation energy.
(iv) Variation of metallic character along a group: Metallic character increases on going down a group from top to bottom. This can be explained in terms of decrease in ionisation energy on going down a group from top to bottom.
Note: Metals generally form cations by losing electrons from their outermost shell, while non- metals generally form anions by accepting one or more electrons.
e.g. Alkali metals form M+ ions by losing one electron, while alkaline earth metals form M–1 ions by losing two electrons from their outermost shell.
Division of Elements into metals and non-metals:
In the modern or long form of periodic table, the elements have been broadly divided into metals and non-metals, by the thick zig-zag line running diagonally across the periodic table
Metals;
Those elements which lie on the left hand side of this line are metals. For example, Na, K, Mg, etc.
Non-Metals:
Those elements which lie on the right hand side of this line are non-metals like P, O, Cl, etc.
Metalliods:
Elements present along the border of this line show properties of both metals and non-metals. For example, Silicon, Germanium, Arsenic, Antimony and Tellurium. These elements are called metalloids or semi-metals.
The oxides of metals are basic in nature while that of non-metals are acidic in nature.