Confused by isomers? You’re not alone.
Thousands of students find this topic tricky—yet isomerism is the hidden key to acing your chemistry exams. Ready to simplify isomers and boost your scores? Discover everything you need to know about isomerism, all in one place.
Why Are Isomers So Important in Chemistry?
Isomerism isn’t just another chapter. It’s the foundation for understanding organic molecules, reactions, and properties that appear in almost every major exam—be it school boards, JEE, NEET, or beyond.
Here’s why mastering isomers matters:
- High-scoring topic in board exams & competitive tests
- Builds a strong base for chapters like hydrocarbons, alcohols, aldehydes, and biomolecules
- Helps you visualize molecular structures and grasp reaction mechanisms
- Essential for practical applications in real-world chemistry and biology
What Will You Learn About Isomers Here?
Our expertly designed content covers every aspect a student needs—from basics to advanced problem-solving. Explore step-by-step guides, clear definitions, solved examples, and exam-oriented tips.
You’ll Get:
- Simple Explanations: Understand what isomerism means, with real-life analogies and easy diagrams
- Types of Isomers: Learn structural, functional, geometric, and optical isomerism—no more confusion!
- How to Identify Isomers: Actionable tricks, common mistakes, and solved questions
- Exam Strategies: Past-year questions, sample papers, and scoring techniques
- Visualization Tools: Interactive images and video breakdowns for difficult concepts
- Practice Sets: Quiz yourself and track your progress in real time
Who Is This Isomers Resource For?
Whether you’re:
- A Class 11 or 12 student revising for finals
- A JEE/NEET aspirant aiming for top ranks
- Someone who just wants to build a solid chemistry foundation
This page is your all-in-one toolkit to master isomerism.
Real Student Success Stories
“I used to dread isomerism questions. After following these guides, I started solving even the trickiest ones with ease!”
— Ananya, Class 12, Board Topper
“The practice quizzes and visual models made a huge difference for my NEET prep. Highly recommended!”
— Arjun, NEET Aspirant
Get Started: Learn Isomers the Easy Way
Ready to transform your chemistry journey?
Start with the basics or jump straight to advanced problem sets. Each lesson is packed with practical tips, memory hacks, and crystal-clear visuals—so you spend less time confused and more time mastering the chapter.
Two or more compounds having same molecular formula but different chemical and physical properties are called isomers and the phenomenon is known as isomerism.
Types of Isomerism
Isomerism can be classified as follows:

Structural Isomerism
Compounds having the same molecular formula but different structural formula, i.e. different arrangement of atoms within the molecule are called structural isomers and the phenomenon is called structural isomerism.
1. Chain or nuclear isomerism: Compounds having the same molecular formula but different arrangement of atoms within the molecule are called chain or nuclear isomers.
Example:

2. Position isomerism: Compounds which differ in the placement of functional (or placement of multiple bonds) group in the carbon chain.
Example:
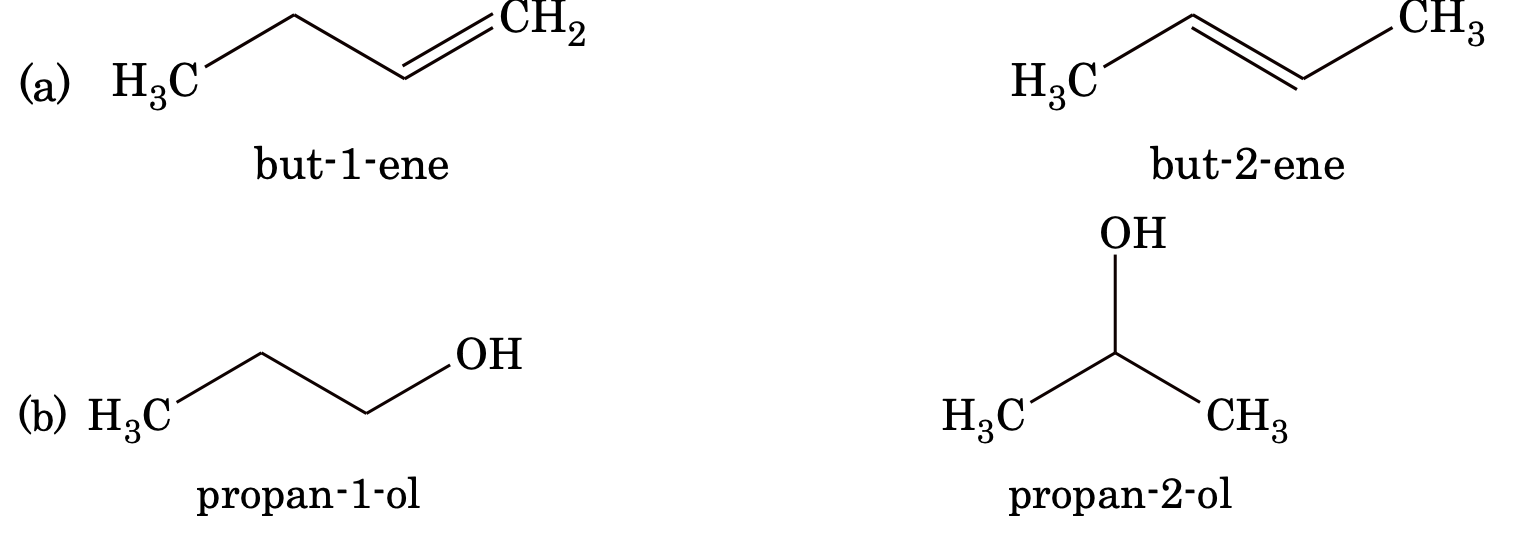
3. Functional isomerism: Compounds having same molecular formula but having different functional groups are called functional isomers.
Example:
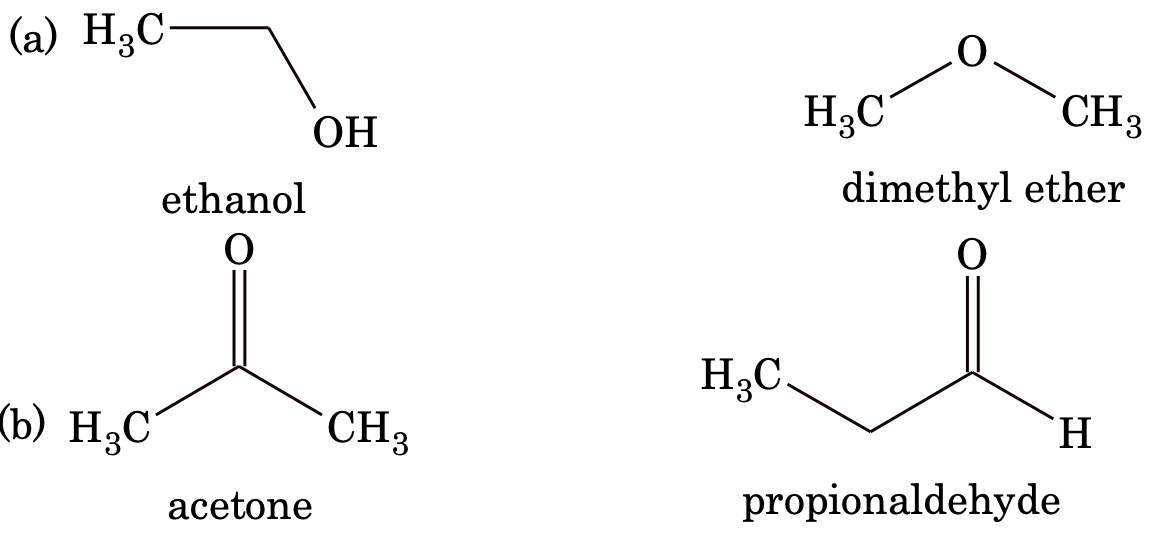
Other pair of compounds which exhibit functional isomerism are (1) carboxylic acid and ester, (2) dienes and alkynes, (3) nitroalkanes and alkyl nitrites, and (4) cyanides and isocyanides.
4. Metamerism: Compounds having same molecular formula but different number of carbon atoms on either side of the functional group are called metamers.
Example:
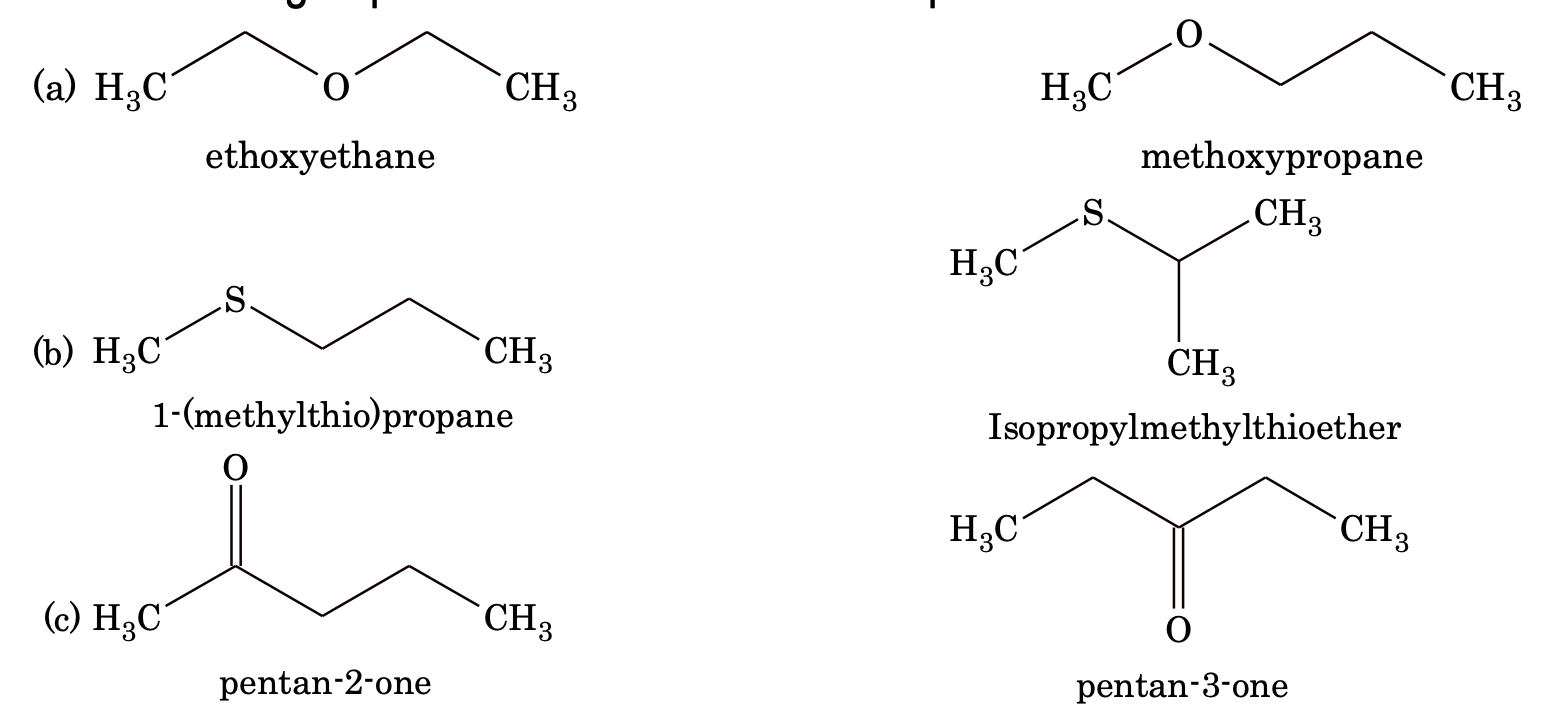
5. Tautomerism: Tautomerism is a type of functional isomerism but in tautomerism the isomers are in dynamic equilibrium with each other. There are several types of tautomerism.
(i) Keto–enol tautomerism
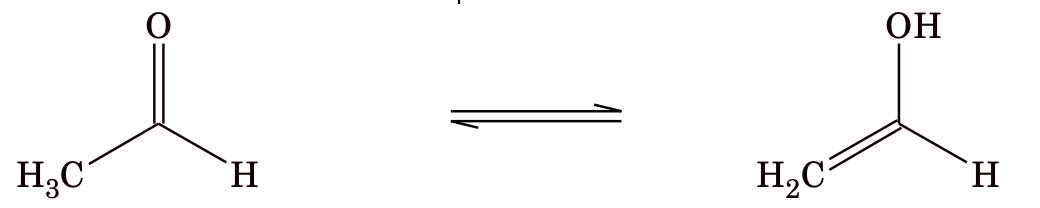
Generally, keto form is more stable than enol form because of the fact that C=O bond is stronger than C=C bond, but there are some exceptions to this rule.
Example:
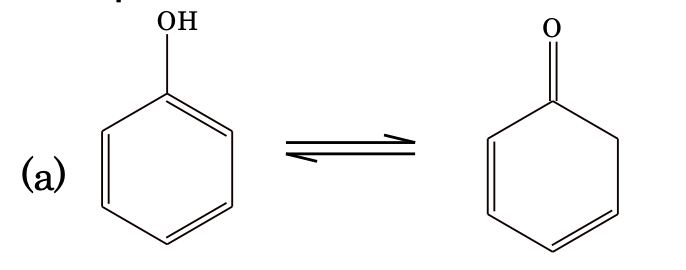
Reason: Phenol gets aromatic stabilization.
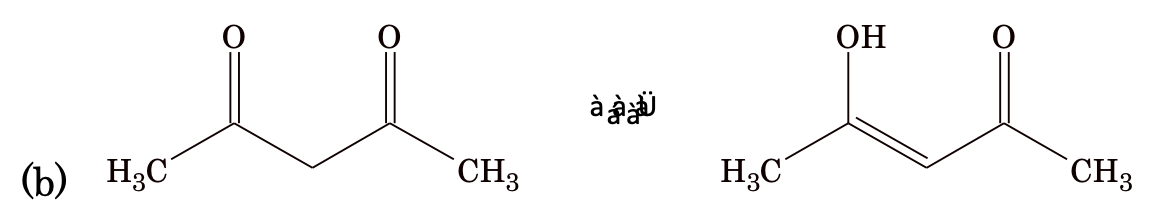
Reason: Extended conjugation and intramolecular hydrogen bonding in enol form.
Effect of solvent: Polar protic solvents such as H2O, CH3OH etc. which form H– bonds with the carbonyl group of the keto form, decrease the enol content. On the other hand, aprotic solvents such as hexane, benzene etc. increases the enol content. For example, enol form of acetylacetone is available as 76% in ethanol but 92% in benzene.
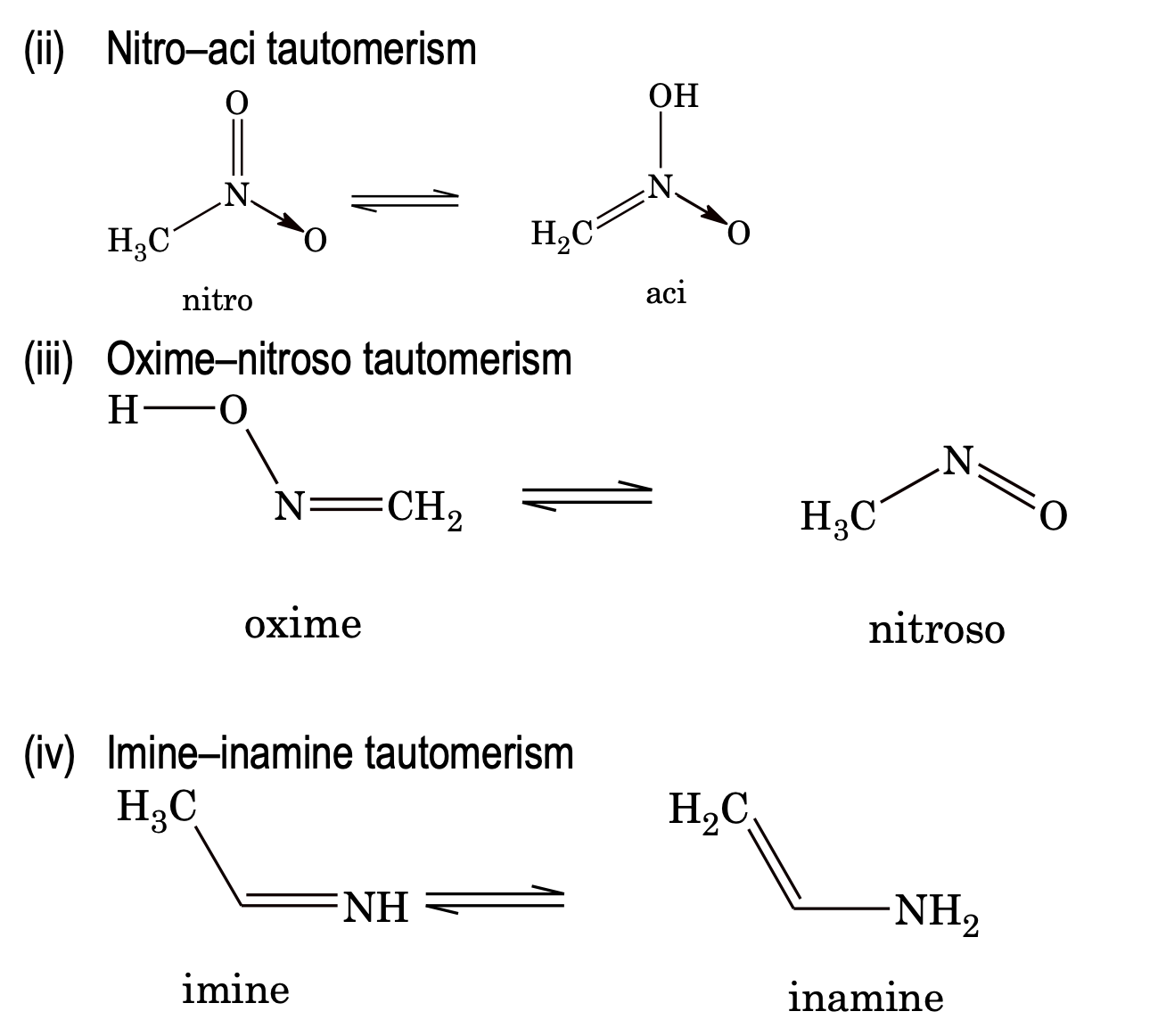
Stereoisomerism
Isomers which have the same structural formula but have different relative arrangement of atoms or groups in space are called stereoisomers.
- Geometrical Isomerism: Many substituted alkenes can exist in two distinct isomeric forms which differ only in the relative position of atoms or groups in space around the double bonds.

The isomer, in which similar atoms or groups lie on the same side of the double bond, is called cis isomer whereas the isomer, in which similar atoms or groups lie on different sides is called trans isomer.
Geometrical isomerism is found whenever there is a restricted rotation about C–C bond. So, geometrical isomerism is possible in cycloalkanes also.
Example:
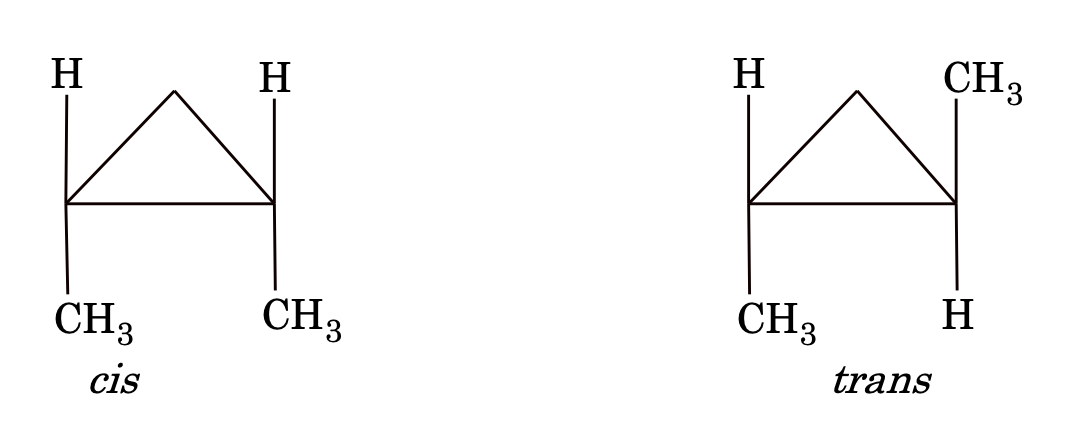
The difference between cis and trans isomers
(i) trans isomer is more stable than cis because of symmetry.
(ii) generally, trans isomers have either zero or less dipole moment than cis isomers.
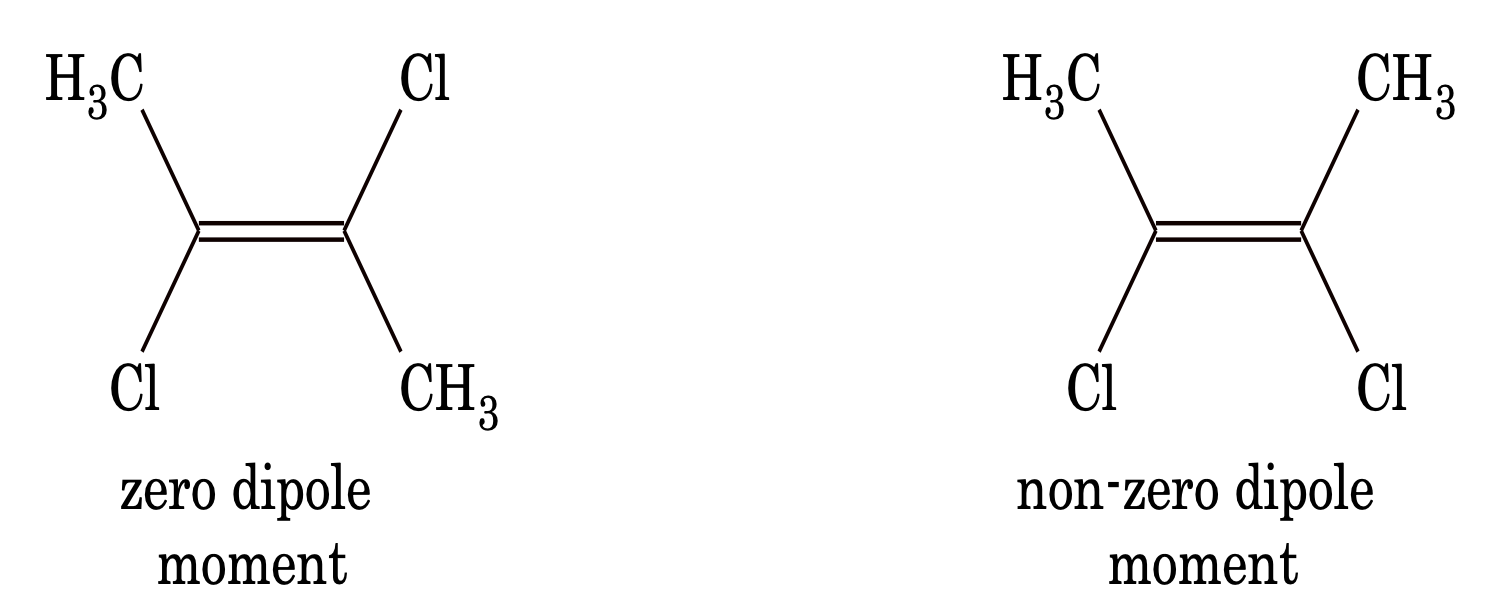
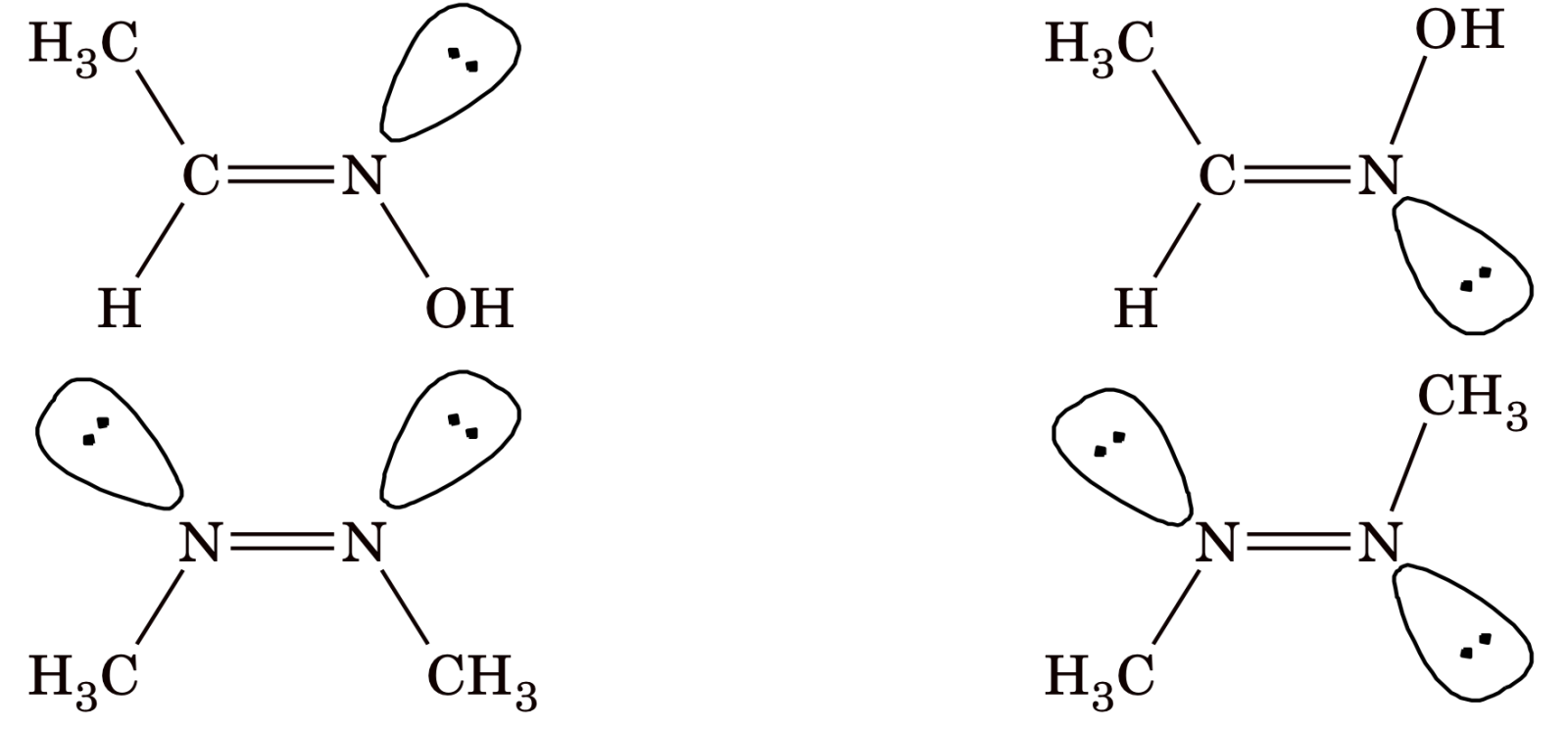
Some other examples of geometrical isomerism are,
2. Optical Isomerism: Compound which rotates the plane of polarized light is known as optically active compound and the phenomenon is known as optical activity. If a substance rotates the plane of polarized light in clockwise direction then it is dextrorotatory (+) form or (d) form. And if a substance rotates the plane of polarized light in anti-clockwise direction then it is laevorotatory (-) form or (l) form. (d) form and (l) form can only be distinguished by the use of a polarimeter and not by the configuration of the compound.
Specific rotation: The specific rotation of an optically active substance is defined as the degrees of rotation observed when the plane polarized light is passed through a tube having a path length of 1 decimetre (10 cm) and a concentration of 1 g/mole of the compound at a specified temperature and wavelength. It is denoted by, where t is the temperature and D is the wavelength of light used.
The specific rotation is calculated as,
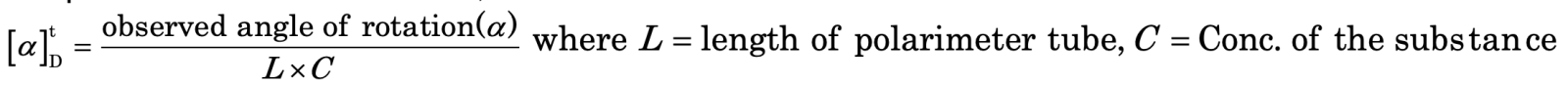
Chirality and asymmetric carbon: A molecule (or an object) is said to be chiral or dissymmetric, if it is not superimposable on its mirror image and the property of non- superimposability is called chirality.
On the other hand, a molecule (or an object) which is superimposable on its mirror image is called achiral (non-dissymmetric or unsymmetric).
Example: Alphabet P is chiral and A is achiral.
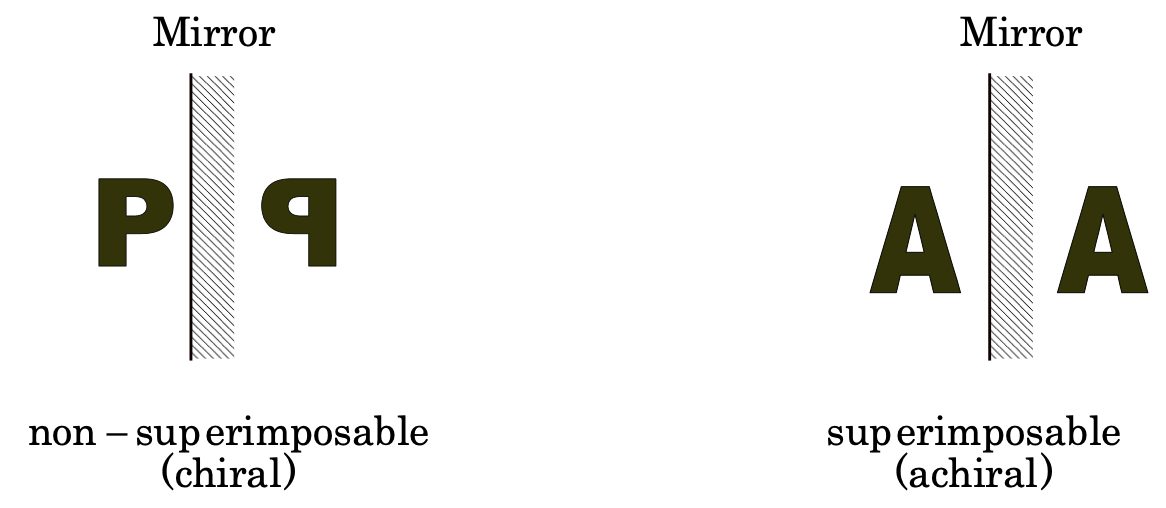
For a substance to be optically active, it must be non-superimposable on its mirror image.
Chiral carbon atom (chiral centre/stereo centre): Carbon atom bonded to four different atoms or groups is called an asymmetric carbon atom or a chiral atom. A chiral atom is indicated by an asterisk.
Example:
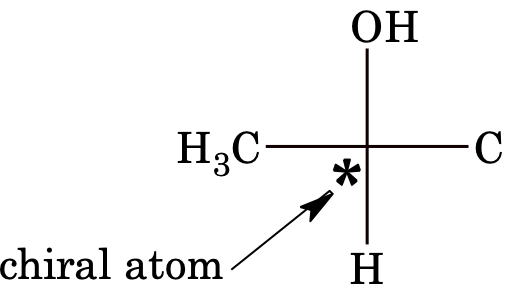
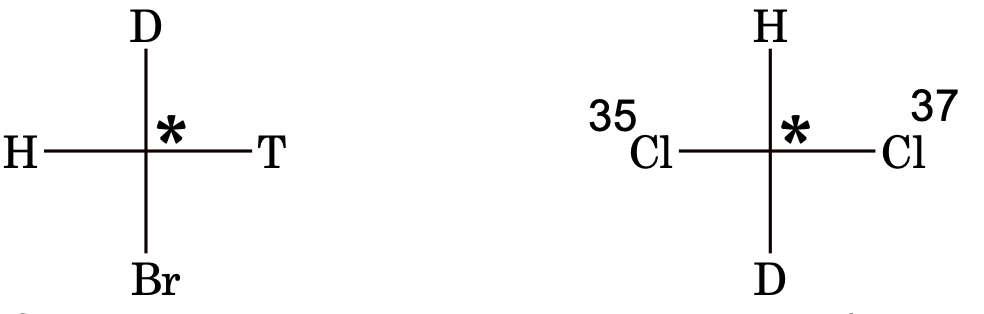
[Note: Isotopes of atoms behave as different groups in stereoisomerism.]
If a molecule contains only one chiral centre/atom, the molecule has to be optically active (i.e. non-superimposable on its mirror image) as it will not contain any element of symmetry. Molecules containing two or more chiral centres may or may not be chiral (optically active). It is necessary to distinguish chirality and chiral centre.
ELEMENTS OF SYMMETRY
- Plane of symmetry
- Centre of symmetry
- Alternate axis of symmetry
ENANTIOMERS
Optical isomers which are non-superimposable mirror images of each other are called enantiomers. The enantiomers have identical physical and chemical properties but rotate the plane of polarized light in opposite directions but to same extent.
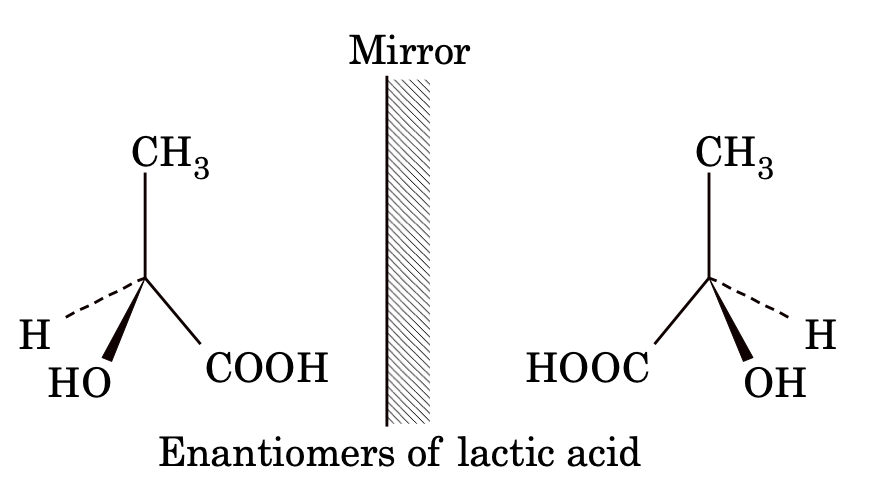
DIASTEREOMERS
The stereoisomers which are not mirror images of each other are called diastereomers. The properties of diastereomers are different from those of enantiomers (which are mirror image of each other).
Example: 2, 3-dibromopentane
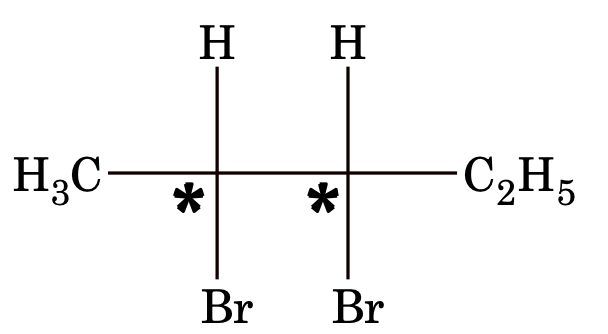
No. of optical isomer possible = 2n = 22 = 4
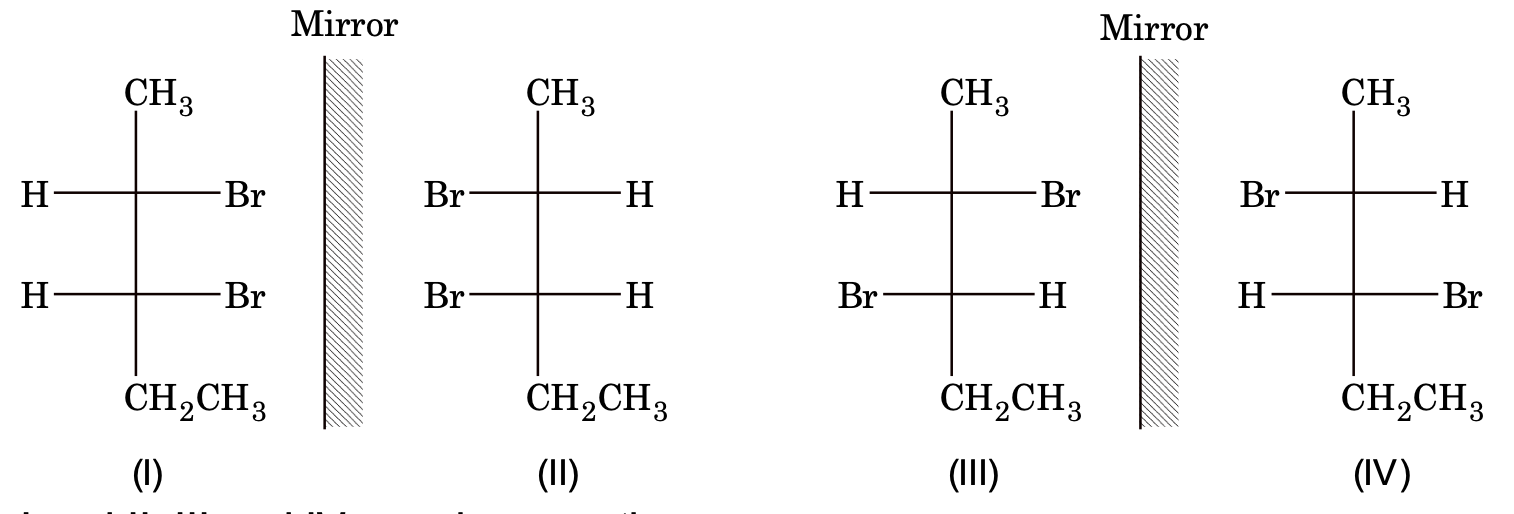
I and II, III and IV are also enantiomers
I and III, I and IV, II and III, II and IV are diastereomer pairs.
MESO COMPOUND
An optically inactive compound whose molecule is superimposable on its mirror image inspite of the presence of chiral carbon atoms, is called a meso compound.
Example: Tartaric acid
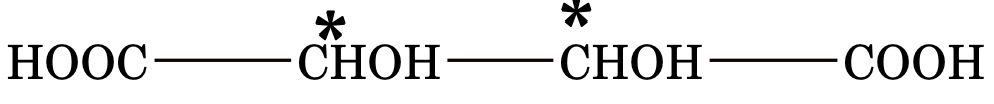
The molecule contains two chiral carbon and the number of optical isomers should be 2n = 22 =4 but the number of optical isomer reduces to 3 because one molecule has plane of symmetry.
Stereoisomers of tartaric acid
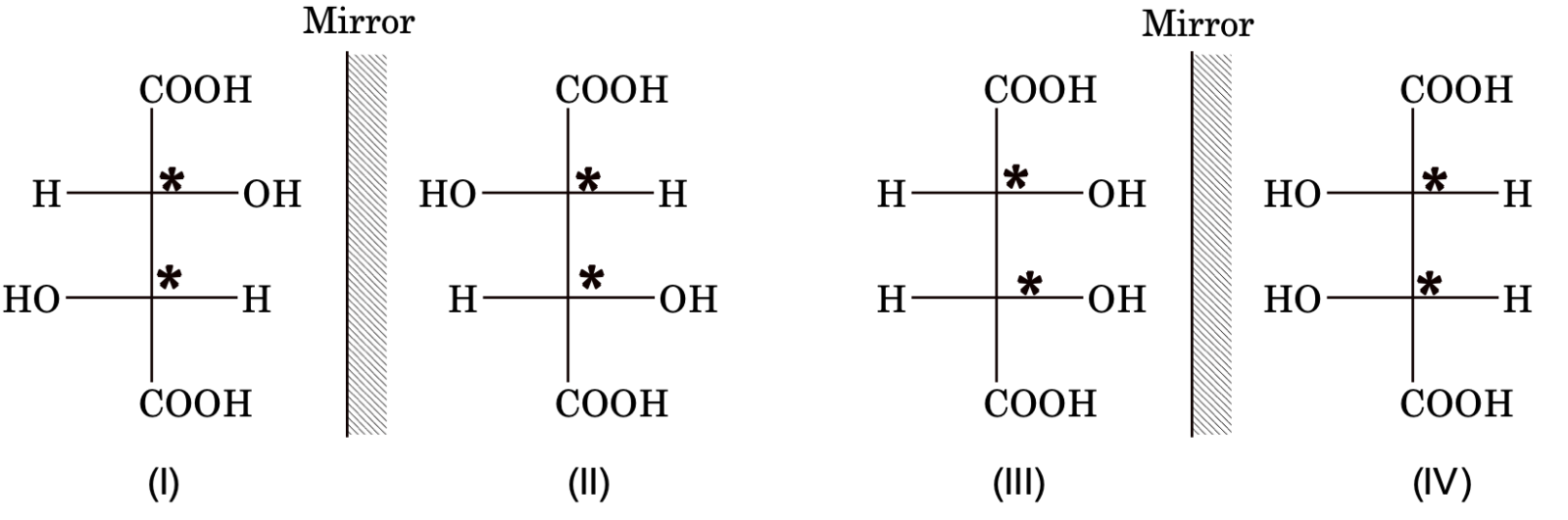
I and II are enantiomers (non-superimposable).
III and IV are meso form (superimposable).
Calculation of number of optical isomers in compounds (containing ‘n’ chiral atoms)
|
S. No. |
Compounds |
No. of optical active forms (a) |
No. of meso forms (m) |
No. of racemic mixture (a/2) |
Total no. of optical isomers |
|
1. |
The molecule has no symmetry |
2n |
0 |
2n−1 |
a+m |
|
2. |
The molecule has symmetry |
||||
|
(a) |
Case1 when compound has even no. of chiral carbon atom |
2n−1 |
2n/2−1 |
2n−1 /2 |
a+m |
|
(b) |
Case2 When compound has odd number of carbon atoms |
2n−1-2(n−1)/2 |
2(n−1)/2 |
2n−1 |
Example:
(i)

No. of optically active forms = a = 23 = 8
(ii)

Number of optical isomer = a = 22−1 = 2
Number of meso form = m = 2n/2−1 = 22/2−1 = 20 = 1
Total number of configuration isomer = 2+1 = 3
(iii)

OPTICAL INACTIVITY DUE TO COMPENSATION
The optical inactivity is also possible due to compensation, i.e.
(a) internal compensation
(b) external compensation.
Internal Compensation
If the rotation of polarized light caused by one half of the molecule is exactly cancelled by equal and opposite rotation caused by the other half of the molecule and the molecule becomes optically inactive. The optical inactivity arises due to internal compensation (within the molecule).Example: Mesotartaric acid
External Compensation
If two enantiomers are mixed together in equimolar amount then the mixture becomes optically inactive. The rotation caused by one enantiomer is exactly cancelled by other enantiomers and is due to external compensation. The resulting optically inactive mixture is called racemic mixture. Example: Equimolar amount of d and l form of tartaric acid.
Resolution: The process by which a racemic mixture can be separated into its (d) and (l) enantiomers is called resolution.
NOMENCLATURE
The arrangement of atoms or group of atoms, which characterizes a particular stereoisomer, is called its configuration. The two terms which are commonly used to describe the configuration of different stereoisomers are absolute and relative configuration.
By absolute configuration we mean the actual arrangement of atoms or group of atoms in space of a particular stereoisomer of a compound.
The relative configuration means the arrangement of atoms or group of atoms in space of stereoisomer of compound relative to the atoms or group of atoms of another compound chosen as arbitrary standard.
D, L Nomenclature or D,L System
Before 1951, there was no method available for determining the absolute configuration of a compound. So, the relative configurations were established with respect to glyceraldehyde chosen as the arbitrary standard. The two enantiomers of this compound were designated D and L symbols. In this, all sugars whose Fischer projection formula shows the –OH group on the chiral carbon atom adjacent to the terminal CH2OH group on the right hand belong to the D–series. Example:
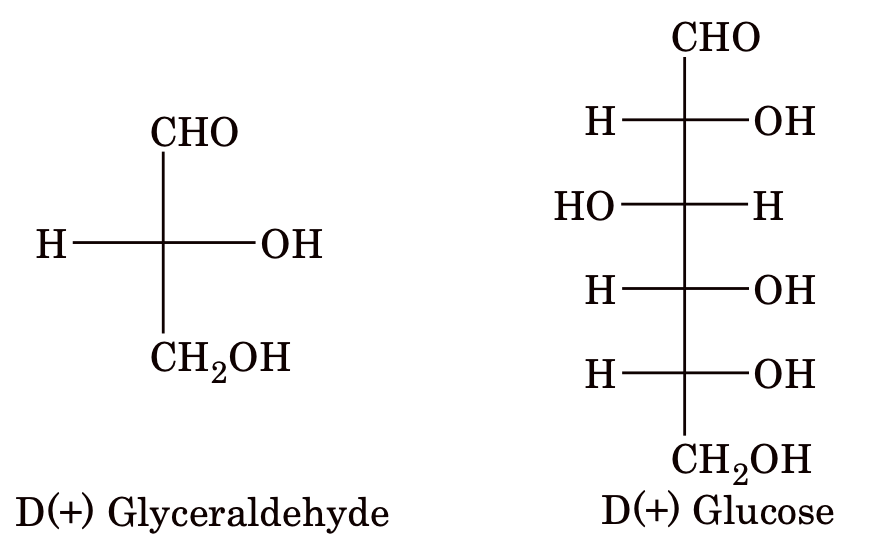
Similarly, if –OH is on the left hand side, then the sugar belongs to L–series.
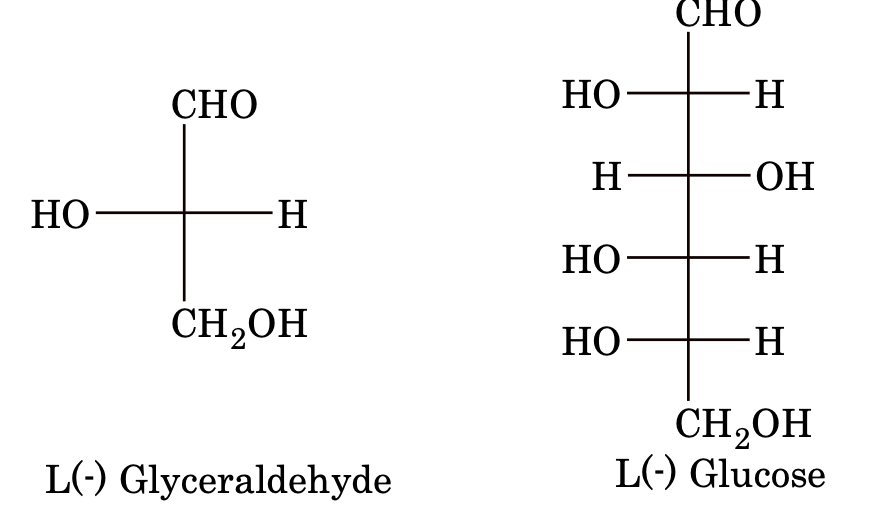
It is important to mention here that there is a relation between configuration and sign of rotation.
Number of optical isomer = 23−1 – 2(3−1)/2 = 22 – 21 = 4−2 = 2
Number of meso form = 2
Total number of optical isomers = 2 + 2 = 4