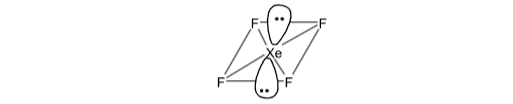
What is Chemical Bonding
A chemical bond is defined as a force that acts between two or more atoms to hold them together as a stable molecule. Atoms combine chemically for the following reasons:
(i) Decrease in energy: Bonded state has lower potential energy than nonbonded state, hence more stability.
(ii) Stable electronic configurations: Every atom tries to attain noble gas configuration, i.e. complete its octet and attains stability (Lewis concept).
Electronic Theory of Valanecy
Combining capacity of an element was initially called as its valency. It depends mainly on the number of electrons present in outermost orbit (i.e. valence electrons). It is the tendency of all other atoms to achieve noble gas electronic configuration (i.e. to achieve 8 electrons in the outermost orbit termed as octet rule). There are two ways by which the atoms can acquire noble gas configuration.
(i) By losing or accepting electrons.
(ii) By sharing electrons.
The Ionic Bond
The chemical bond formed by the complete transfer of one or more electrons from one atom to the other, e.g. NaCl, LiF, MgBr, MgO etc.
Conditions for forming ionic bond.
(i) Low ionization potential
(ii) High electron affinity
(iii) High lattice energy of the compound.
The summation of the above three energies should be negative, i.e. energy is released.
I.P. + E.A. + L.E. = -ve (Born Hayber cycle)
Characteristics of ionic compounds.
(i) They have high melting and boiling points. (Due to coulombic force)
(ii) These are highly soluble in polar solvents.
(iii) Ionic reactions are quite fast and instantaneous.
(iv) Isomorphism: Crystals of different ionic compounds having same geometry are known as isomorphs.
(v) If electronegativity difference will >1.7, between bonding atom than ionic character will dominant.
The Covalent Bond
The bond formed by the mutual sharing of the electrons by different or same type of atoms,e.g.N2, H2, CO2, CH4 etc.
Characteristics of covalent compounds.
(i) These compounds are molecular in nature and hence are bad conductors of electricity.
(ii) They have low melting and boiling points.
(iii) These are soluble in non-polar solvents like benzene (like dissolve like).
(iv) They can show structural and space isomerism.
(v) Their reactions which are quite slow and complex.
Co-Ordinate Covalent or Dative Bond
These are special type of covalent bonds where both the electrons forming the bond are contributed only by one atom but are shared by both the atoms, e.g. O3, NH4Cl, H2SO4 etc. Their properties are intermediate between ionic and covalent compounds.
The bond is represented by an arrow (↑).
(a) O3
O = O ⟶ O (b) H2SO4 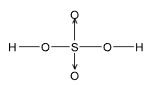
Exceptions to the Octet Rule
(i) Incomplete octet: Examples are BeCl2, BF3, diborane etc.
(ii) Expanded octet: Examples are PCl5, ClF3, SF6 etc. P(in PCl5) and Cl (in ClF3) have 10 electrons and S (in SF6) have 12 electrons.
(iii) Odd electron bonds: Examples of compounds having unpaired electron(s) are NO, NO2, ClO2, O-2, etc.
Bond Characteristics
Bond length. The average distance between the nuclei of two atoms bonded to each other is called bond length.
Bond energy. The enthalpy change required to break a particular bond in 1 mole of gaseous molecule is called bond energy.
Bond angle. The angle between the orbitals containing the bonding electrons is called the bond angle.
Example 1 : The bond in NH4Cl are
(A)only ionic
(B) ionic and covalent
(C)coordinate bond and ionic bond
(D) ionic,covalent& coordinate bond
Solution: (D).
Three H atom makes covalent bond with N and one H make coordinate bond and NH+4 cation make ionic bond with Cl anion

Dipole Moment
If a covalent bond is formed between different atoms then the shared pair is displaced towards the more electronegative atom. Such a covalent bond is called polar bond and polarity is defined by dipole moment. For a diatomic molecule, the product of the magnitude of the positive or negative charge on each atom of the molecule and the distance between the centres of the two atoms is called dipole moment.
i.e. 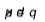
where, μ is the dipole moment, q is the charge and d is the distance.
Its unit is Debye, i.e. 10–18 e.s.u. cm
The dipole moment of any molecule can be determined experimentally. The value provides a measure of the polar character of the molecule. The dipole is a vector quantity and is represented by the symbol 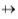 pointing from the positive centre towards the negative centre.
pointing from the positive centre towards the negative centre.
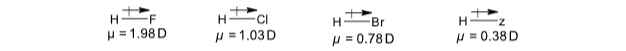
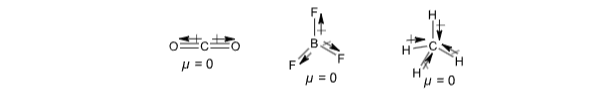
For polyatomic molecules, the dipole moment is the vector sum of the dipole moments of various bonds. If the molecule is symmetrical, the resultant dipole moment of the molecule is zero. For example, CO2, BF3, CH4 etc.
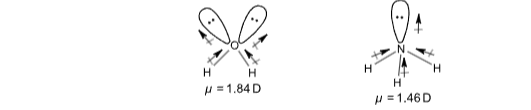
Unsymmetrical molecules have a resultant dipole moment. For example, H2O, NH3 etc.
Applications of dipole moment.
(i) The dipole moment helps to predict whether the molecule is polar or non-polar.
(ii) It can also predict whether molecule is symmetrical or not by resultant dipole of complete molecule.
(iii) The % age ionic character can also be calculated as follows:
% age ionic character 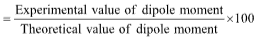
Polarizing Power and Polarizability (Fajan’s Rule)
The ability of a cation to polarize the near by anion is called its polarizing power and the tendency of an anion to get distorted or polarized by the cation is called its polarizability. Due to polarization, sharing of electrons occur between two ions to some extent and the bond shows some covalent character.
The factors increasing the covalent character are
(i) small positive ions (cations): Li+ > Na+ >K+ >Rb+ >Cs+
(ii) large negative ions (anions): I- >Br- >Cl- >F-
(iii) large charge on either of the two ions: Na+Cl- > Mg2+(Cl2)2- > Al3+(Cl3)3-
(iv) configuration of the cation: 18 electrons in outermost shell (pseudo noble gas configuration) bring greater polarization than 8 electrons (noble gas configuration).
Ex.: Which of the following molecules has non – zero dipole moment?
(A) PCl5
(B) BF3
(C) SO2
(D) CCl4
Solution: (C).
SO2, being a bent molecules has non – zero dipole moment

VALENCE BOND THEORY
According to this concept there is a condition of maximum atomic orbital overlap leading to maximum bond strength at a particular internuclear distance (bond length).
(i) The covalent bond is a region of high electron charge density that result from the overlaps of atomic orbitals between two atoms.
(ii) Two types of bonds are form after overlapping viz σ and π bond.
(iii) If head on overlapping take place then strong σ bond will form.
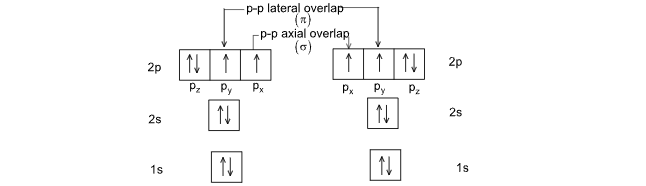
Ex: H2 molecule. H-H σ bond is formed by S-S overlapping.
(iv) Lateral overlapping result in the formation of π bond.
Ex: O2 molecule. One σ bond is by axial overlap of p-orbitals and π bond is by lateral overlap of p-orbitals.
Hybridization of Atomic Orbitals
It is defined as the concept of intermixing of orbitals of same energy or of slightly different energy to produce entirely new orbitals of equivalent energy and symmetrically disposed in plane. New orbitals formed are called hybrid orbitals. Only orbitals of similar energies and belonging to the same atom or ion undergo hybridization. Hybridized orbitals show only head-on overlapping and thus form only sigma bonds. They never form π bonds.
Different types of hybridization lead to different orientation in space.
Hybridization Table
| Type of hybridization | Orbitals used | Orientation of hybrid orbitals | Examples |
|---|---|---|---|
| sp | one s and one p | Linear | BeF2, BeCl2, CO2 |
| sp2 | one s and two p | Trigonal planar | BF3, SO2, SO3, C2H4 |
| sp3 | one s and three p | Tetrahedral | CH4, CCl4, SF4, H2O, NH3 |
| sp3d | one s, three p and one d | Trigonal bipyramidal | XeF2, ClF3, SF4, PCl5 |
| sp3d2 | one s, three p and two d | Octahedral | XeF4, BrF5, SF6 |
| sp3d3 | one s, three p and three d | Pentagonal bipyramidal | IF7 |
VSEPR THEORY (VALENCE SHELL ELECTRON PAIR REPULSION THEORY)
VSEPR theory helps us in explaining the shapes of molecule having lone pair of electrons. The main points of VSEPR theory are
(i) The shape of the molecule depends on the number of electron pairs present in the valence shell or outermost shell of the central atom.
(ii) The electron pairs present in the central atom can be regarded as point charge in space.
(iii) The electron pairs orient in space so as to have minimum repulsion among them.
(iv) The order of repulsions among various electron pairs is
lone pair – lone pair > lone pair – bond pair > bond pair – bond pair
(v) The most stable geometrical arrangement for 2, 3, 4, 5 and 6 electron pairs is linear, trigonal planar, tetrahedral, trigonal bipyramidal and octahedral respectively.
The presence of lone pairs at the central atom causes distortion in regular shapes and decrease in bond angles. Presence of π bonds may be ignored if present at the central atom.
Depending upon the number of bond pairs and lone pairs, the geometry of various types of molecules are summarized below:
Molecules Table
|
Type of Type of molecules |
Total no. of electron pairs |
No. of bond pairs |
No. of lone pairs |
Type of hybridization involved |
Geometry of molecule | Examples |
|---|---|---|---|---|---|---|
| AB2 | 2 | 2 | 0 | sp | Linear | BeF2,[Ag(NH3)2]+ |
| AB3 | 3 | 3 | 0 | sp2 | Trigonal planar | BF3, AICI3+, NO-3, CO2-3 |
| AB2L | 3 | 2 | 1 | sp2 | V shaped | SnCl2, PbCl2 |
| AB4 | 4 | 4 | 0 | sp2 | Tetrahedral | CH4, SiF4, NH+4, CCl4 |
| AB3L | 4 | 3 | 1 | sp3 | Trigonal bipyramidal | NH4, Px3 |
| AB2L2 | 4 | 2 | 2 | sp3 | V shaped | H2O, OF2, SbCl2 |
| AB5 | 5 | 5 | 0 | sp3d | Trigonal bipyramidal | PF5, PCl5, SbCl5 |
| AB4L | 5 | 4 | 4 | sp3d | Sea saw | SF4, TeBr4 |
| AB3L2 | 5 | 3 | 2 | sp3d | T shaped | CIF3, XeOF3 |
| AB2L3 | 5 | 2 | 3 | sp3d | Linear | XeF2, I-3, ICI-2 |
| AB6 | 6 | 6 | 0 | sp3d2 | Octahedral | SF4, SeF6 |
| AB6L | 6 | 5 | 1 | sp3d2 | Square pyramidal | IF5, CIF6, XeOF4 |
| AB4L2 | 6 | 4 | 2 | sp3d2 | Square planar | SF4, XeF4 |
| AB7 | 7 | 7 | 0 | sp3d3 | Pentagonal bipyramidal | IF7 |
Ex.: The hybrid state of S in SO3 is similar to that of
(A) C in C2H2
(B) C in C2H4
(C) C in CH4
(D) C in CO2
Solution: (B).
S in SO3 and C is C2H4 have sp2 hybridisation
Ex.: The bond angles of NH3, NH4+ and NH2- are in the order
(A) NH2- > NH3 > NH4+
(B) NH4+ > NH3 > NH2-
(C) NH3 > NH2- > NH4+
(D) NH3 > NH4+ > NH2-
Solution: (B)
Bond angles NH4+ > NH3 > NH2-. This is because NH4+ has no lone pair, NH3 has one lone pair while NH2- has two lone pairs to repel the bond pairs.
MOLECULAR ORBITAL THEORY
The modern researches based on the concept of wave mechanics proposed molecular orbital theory. This new approach assumes that when atomic orbitals approach each other their wave functions interact with each other in two different ways, i.e.
(i) constructive interference (or addition overlap)
(ii) destructive interference (or substraction overlap)
Thus, according to this theory, all the atomic orbitals or the atoms participating in bonding are confined and changed into molecular orbitals. In doing so, the atomic orbitals looses its individual character and all the electrons in a molecule are present in molecular orbitals. The atomic orbitals are confined in pairs and each pair of atomic orbitals gives rise to molecular orbitals of two types, viz. (a) bonding molecular orbitals (σ or π) and (b) antibonding molecular orbitals (σ* or π*) . The bonding molecular orbitals are of lower energy than the combining atomic orbitals whereas the antibonding molecular orbitals are of higher energy than the combing atomic orbitals.
The filling of molecular orbitals in a molecule takes place in accordance with Aufbau principle, Pauli’s exclusion principle and Hund’s rule. The general order of increasing energy among the molecular orbitals formed by the elements of second period and hydrogen and their general electronic configurations are given below:
For species like O2, O+2, O-2, F2, Ne2 etc. the energy of the orbitals is as follows:
For molecules like B2, C2 and N2 the energy of the orbitals is as follows:
σ1s < σ*1s < σ2s <σ*2s <π2px = π2py < σ2pz < π*2px = π*2py <σ*2pz
Bond Order
Bond order (B.O.) = 1/2 (Nb - Na)
where Nb = number of electrons in the bonding molecular orbitals
Na = number of electrons in the antibonding molecular orbitals
Bond order helps to predict:
(i) Formation of molecule/molecular ions——if bond order is greater than zero, then the molecules exist otherwise not.
(ii) Stability——higher the bond order, greater is the stability.
(iii) Bond dissociation energy——higher the bond order, higher is the bond energy.
(iv) Bond length——higher the bond order, shorter is the bond length.
(v) If any compound show resonance then effective bond order can be calculated as
Bond order = Total number of bonds between atoms / Total number of resonating structures
Ex.: The bond energies in NO. NO+ and NO- follows the order
(A) NO+ > NO > NO-
(B) NO > NO+ > NO-
(C) NO- > NO > NO+
(D) NO+ > NO- > NO
Solution: (A). B.O. in NO = 1/2 (10 – 5) = 2.5
B.O. in NO+ = (10 – 4) = 3.0
B.O. in NO- = (10 – 6) = 2.0
Since the bond energies are directly related to bond order. Therefore, the correct order of increasing bond energies is
NO+ > NO > NO-
Ex.: In which set of molecule are all the species paramagnetic?
(A) B2, O2, N2
(B) B2, O2, NO
(C) B2, F2, O2
(D) B2, O2, Li2
Solution: (B).
B2 has two, O2 has two and NO has one unpaired electron and hence all these molecules are paramagnetic.
Hydrogen bonding
Hydrogen bonding is said to be formed when a slightly acidic hydrogen attached to a strongly electronegative atom such as F, N and O, is held with weak electrostatic forces by the non bonded pair of electrons of another atom. Of all the electronegative donor atoms, only F, N and O atoms enter into stable hydrogen bond formation.
Types of H-bonding
Intramolecular H-bonding.
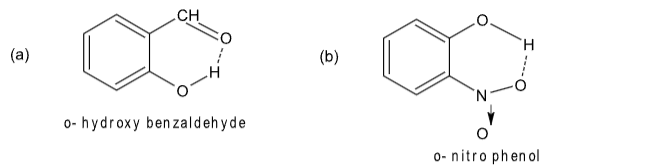
Intermolecular H-bonding.
(a) In water molecules
Due to polar nature of H2O, there is association of water molecules giving a liquid state of abnormally high boiling point.

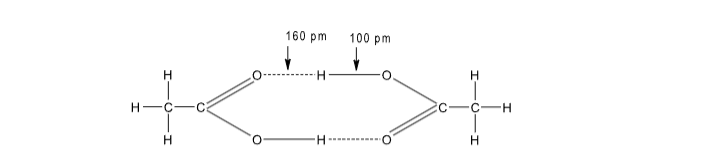
(b) Carboxylic acid dimerises in gaseous state due to H-bonding.
Consequences of H-bonding.
(i) Boiling points of water, ammonia, hydrofluoric acid are abnormally high in their group due to H-bonding.
(ii) Boiling points of alcohols are higher than that of corresponding ethers.
(iii) Solubility of the organic compounds in water is due H-bond formation.
(iv) Viscosity (η) of the liquid increases due to H-bonding.
Points to Remember
1. Chemical bond is a force that acts between two or more atoms to hold them together as a stable molecule.
2. Valency of an element depends mainly on the number of electron present in the outermost shell.
3. Favourable conditions for forming ionic bond are
(i) low ionization potential
(ii) high electron affinity
(iii) high lattice energy of the compound
4. Crystals of different ionic compounds having same geometry are known as isomorphs.
5. Covalent bond is formed by the mutual sharing of the electron by different or same type of atoms.
6. Covalent compounds show structural and space isomerism.
7. Dative bond is a special type of covalent bond where both the electrons forming the bond are contributed by one atom but are shared by both the atoms.
8. For a diatomic polar molecule, the product of the magnitude of the positive or negative charge on each atom of the molecule and the distance between the centres of the two atom is called dipole moment.
9. The ability of a cation to polarize the nearby anion is called its polarizing power and the tendency of an anion to get distorted or polarized by the cation is called its polarizability.
10. According to valence bond theory, there is a condition of maximum atomic orbital overlap leading to maximum bond strength at a particular internuclear distance (bond length).
11. Hybridization of atomic orbitals is the concept of intermixing of orbitals of same energy or of slightly different energy to produce entirely new orbitals of equivalent energy and symmetrically disposed in space. New orbitals thus formed are called hybrid orbitals.
12. VSEPR theory helps us in explaining the shapes of molecules having lone pair of electrons.
13. Molecular orbital theory is based on the concept of wave mechanics.
Solved Examples
1. Which of the following is an example of super octet molecule?
(A) CF3
(B) PCl5
(C) IF7
(D) All the three
Sol. (D). All the molecules are supper octet molecule since in all these molecules; the central atom has more than 8 electrons
2. HCl is a gas but HF is low boiling liquid because
(A) H-F bond is weak
(B) HCl bond is strong
(C) HF is a weak acid
(D) Hydrogen bonding
Sol. (D).
Hydrogen attached to electronegative atoms like F, O, N shows hydrogen bonding. So much compounds shows different characteristics compound, compare to the compound of other group member. HF show H-bonding but HCl not.
3. N2 is chemically inert
(A) presence of large number of bond electrons compare to antibonding electrons
(B) high heat of dissociation
(C) presence of triple bond
(D) all the statements are correct
Sol. (D).
Electronic configuration of 7N = 1s2, 2s22p3
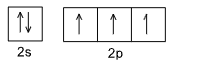
In 2p subshell half filled orbital configuration is present which is stable one. Also nitrogen formed three bond with another nitrogen atom to completes its octet which leads to high heat f dissociation.
4. Which of the following compounds is nonpolar?
(A) CH3Cl
(B) CH2Cl2
(C) CHCl3
(D) CCl4
Sol. (D).All the molecules have polar C–Cl bonds. But in CCl4 due to regular tetrahedral geometry, the four dipole moments cancel each other. Hence CCl4 has zero dipole moment and thus is non-polar.
5. The number and type of bonds between two carbon atoms in CaC2
(A) One s and one p bond
(B) one s and two p bonds
(C) one s and a half p bonds
(D) one s bond
Sol. (B).
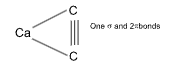
The triple bond between C º C bond is a combination of 1 s 2 p bonds
6. According to M.O.T. bond order of N2 is
( A) 1
(B) 2
(C) 3
(D) 4
Sol. (C). Molecular orbital configuration of N2 is σ1s² σ1s² σ2s² σ2s² π2px² π2py² σ2pz²
σ Bond order = No. of es in bonding orbital - No. of es in antibonding orbital 8/2 = 8.2/2 =3
7. Bond angle is minimum is
(A) H2O
(B) CO2
(C) NH3
(D) CH4
Sol. (A).
In CO2 molecule, structure is linear O = C = O. So bond angle is 180°
In H2O, NH3 & CH4 the central atom is sp3 hybridised
In CH4 4 bond pair of electrons and no lone pair of electron is present.
In NH3 3 bond pair of electron and 1 lone pair of electron is present.
In H2O 2 bond pair of electron and 2 lone pair of electron is present.
Greater is no. of lone pair greater is the lone pair bond pair repulsion smaller is the long angle.
So bond angle is least in case of H2O
8. Which one has a coordinate bond?
(A) Al2Cl6
(B) BF3
(C) NaCl
(D) O2
Sol. (A).
Structure of Al2Cl6
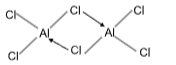
So it has coordinate bond
BF3 has only covalent bond. NaCl is ionic
R O2 has covalent bond
9. What is true about N2 and CN-?
(A) Both are isoelectronic
(B) Both chemically inert
(C) Both are highly reactive
(D) Both have same polarity
Sol. (A).
Number of electrons in N2 = 7 + 7 = 14 and number of electrons in
CN- = 6 + 7 + 1 = 14 So both are isoelectronic.
10. Which of the following is planar?
(A) XeO4
(B) XeO3F
(C) XeO2F2
(D) XeF4
Sol. (D).
Hybridisation of XeF4 = 1/2 [V + M - C + A] = 1/2 [8 + 4 - 0 + 0] = 6
Sp3d2 should be octahedral but it have only four F atom. So it have square planer shape.