Is calculating pH giving you a headache? Are buffer solutions and salt hydrolysis confusing? Stop struggling and start scoring! Get the ultimate CBSE notes for Ionic Equilibrium, designed by FIITJEE experts to make learning simple and effective.
Ionic Equilibrium Confusion
Ionic Equilibrium is a critical chapter for your CBSE and competitive exams, but it's often the one students find most challenging. Do you face these problems?
-
Complex Theories: Juggling Arrhenius, Brønsted-Lowry, and Lewis acid-base concepts.
-
Tricky Calculations: Getting stuck on pH, solubility product (Ksp), and hydrolysis constant (Kh) problems.
-
Forgetting Formulas: Forgetting key equations like the Henderson-Hasselbalch equation during exams.
-
Lack of Practice: Not having enough solved examples and questions to build confidence.
Your Ultimate Solution for Acing Ionic Equilibrium
These aren't just notes; they're your roadmap to mastering the chapter. Crafted by experts from Home Tuition, this guide simplifies everything.
What's Inside?
Our comprehensive notes cover every topic you need to know, logically structured for better understanding.
-
Fundamentals First: Clear explanations of Electrolytes, Degree of Dissociation, and Ostwald's Dilution Law.
-
All Acid-Base Theories: Master the Arrhenius, Brønsted-Lowry, and Lewis concepts with simple examples.
-
pH and Kw Mastery: Deep dive into the pH scale and the Ionic Product of Water (Kw).
-
Buffer Solutions Explained: Understand how acidic and basic buffers work and learn to calculate their pH using the Henderson-Hasselbalch equation.
-
Salt Hydrolysis Demystified: Learn why solutions of salts like CH3COONa are basic or why NH4Cl solutions are acidic.
-
Solubility Product (Ksp): Predict precipitation with the concepts of Ionic Product and Solubility Product.
-
Key Applications: Learn about the Common Ion Effect and its role in qualitative analysis, plus the use of indicators in acid-base titrations.
Why Choose Our Notes?
| Feature | Your Advantage |
| Step-by-Step Solved Examples | Learn the exact method to solve complex numericals and score full marks. |
| Practice Exercises with Answers |
Test your understanding and get exam-ready with a curated set of problems. |
| Expert-Designed Content | Trust notes prepared by Home Tuition faculty, who know how to crack the exams. |
| Clear & Concise Formulas | Get all important formulas in one place for quick and effective revision. |
| Covers All Key Concepts | From basic definitions to advanced applications, we cover everything for CBSE and beyond. |
Don't let Ionic Equilibrium pull your grade down. Get the expert guidance you need to turn your weakness into a strength.
Electrolytes
The compound which gives ions and conduct electricity in molten state or in aqueous solution are called electrolytes.
Strong electrolytes
These electrolytes dissociate almost completely in a polar medium. For example, NaOH, HCl, HNO₃.
Weak electrolytes
These electrolytes dissociates only to a small extent. For example, CH₃COOH, HCN, NH₄OH etc.
Degree of dissociation
It is the fraction of total amount of an electrolyte which dissociated into ions.
Ostwald's dilution law
It states that the degree of dissociation of weak electrolyte is proportional to the square root of dilution.
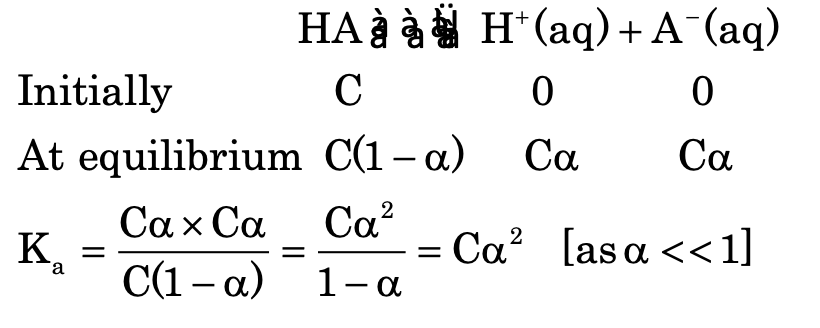
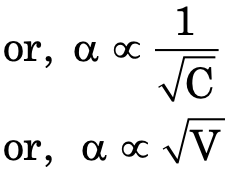
where V = volume of solution containing one mole of solute.
ACID-BASE CONCEPTS
Arrhenius concept of acids and bases
An acid is a substance which gives H⁺ ions in aqueous solution whereas a base is a substance which gives OH⁻ ions in aqueous solution. For example, HCl is an acid and NaOH is a base.
Bronsted Lowry concept of acids and bases
An acid is a substance which gives proton and a base is a substance which accepts a proton. For example, in the reaction:
NH₃ is the base as it accepts proton to form NH₄⁺ and H₂O is an acid as it donates proton.
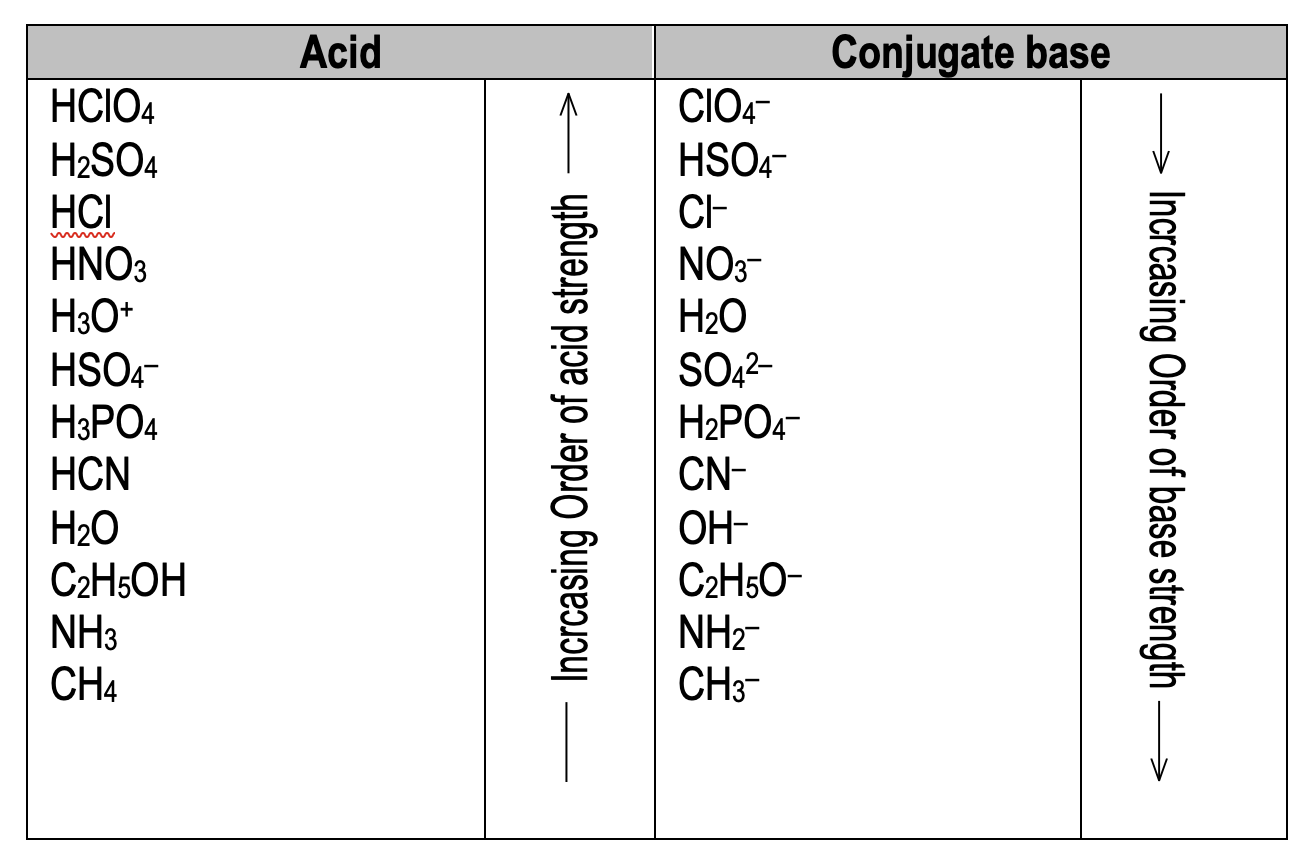
Conjugate acid base pairs
An acid base pair which differ by one proton is called conjugate acid base pair. In the reaction:
acid₁ + base₁ ⇌ base₂ + acid₂
CH₃COO⁻ is conjugate base of CH₃COOH and H₃O⁺ is conjugate acid of H₂O. A strong acid has weak conjugate base and vice versa.
Exercise 1: The correct order of conjugate base strength is
(A) H₃O⁺ > H₂O > OH⁻ > O²⁻
(B) O²⁻ > OH⁻ > H₂O > H₃O⁺
(C) H₂O > H₃O⁺ > O²⁻ > OH⁻
(D) H₃O⁺ > H₂O > O²⁻ > OH⁻
Leveling effect
All acid above H₂O show same acid strength in H₂O. This is called leveling effect. The acid strength of HClO₄, H₂SO₄, HCl, and HNO₃ in water is same but in acetic acid follows the order HClO₄ > H₂SO₄ > HCl > HNO₃.
The relative strength of acids
The relative strength of acids depends upon their degree of ionization (α).
Hence, Strength of acid₁/Strength of acid₂ = √(Ka₁/Ka₂)
Similarly, Strength of base₁/Strength of base₂ = √(Kb₁/Kb₂)
(A) Ka = 10⁻⁶
(B) pKa = 5
(C) pKb = 10
(D) Kb = 10⁻¹¹
Solution: (D). Smaller the Kb value lesser is the basicity and more is the acidity.
Types of Solvents
Protophilic solvents
Solvents which have greater tendency to accept protons. For example, water, liquid NH₃, alcohol etc.
Protogenic solvents or protic solvents
Solvent which have tendency to produce protons. For example, water, HCl, glacial acetic acid.
Amphiprotic solvents
Solvents which act both as protophilic and protogenic, e.g. water, alcohol, NH₃ etc.
Aprotic solvents
Solvents which neither donates nor accepts protons, e.g. benzene, carbon tetrachloride carbon disulphide etc.
Lewis concepts of acid and bases
An acid is a substance which can accepts a pair of electrons and a base is a substance which donates a pair of electrons. For example, BF₃ is a lewis acid and NH₃ is a lewis base.
ACID BASE NEUTRALIZATION–SALTS
Neutralization of a base with acid involves the interaction between OH⁻ and H⁺ ions, e.g.
or, H⁺(aq) + Cl⁻(aq) + Na⁺(aq) + OH⁻(aq) → Na⁺(aq) + Cl⁻(aq) + H₂O(l)
or H⁺(aq) + OH⁻(aq) → H₂O(l)
Simple salt
Salt formed by neutralization of an acid and a base is called simple salt. These are of following types.
(i) Normal salts
Salts which do not contain any replaceable H⁺ or OH⁻ ions are called normal salts, e.g. NaCl, KNO₃ etc.
(ii) Acidic salts
Salts formed by incomplete neutralization of polybasic acids and containing replaceable H⁺ ions called acidic salt, e.g. KHSO₄, NaH₂PO₄ etc.
(iii) Basic salts
Salts formed by incomplete neutralisation of polyacidic bases and containing replaceable OH⁻ ions are called basic salts, e.g. Zn(OH)Cl, Mg(OH)Cl etc.
(iv) Double salts
The compounds formed by combination of two simple salts are called double salts, e.g. Mohr's salt (FeSO₄.(NH₄)₂SO₄. 6H₂O), potash alum (K₂SO₄. Al₂(SO₄)₃.24H₂O) etc.
Mixed salts
The salt which furnishes more than one cation or anion in solution is called mixed salt, e.g. Ca(OCl)Cl, KNaSO₄ etc.
IONIC PRODUCT OF WATER
It is defined as the product of molar concentrations of H₃O⁺ and OH⁻ ions, i.e. Kw = [H₃O⁺] [OH⁻]. Its value is 10⁻¹⁴ at 25°C and increases with increase of temperature as dissociation of H₂O increases.
(A) 10⁻¹⁴/12.67
(B) 10⁻¹⁴ ×10⁻¹²·⁶⁷
(C) 10⁻¹⁴/10⁻¹²·⁶⁷
(D) 10⁻¹⁴/10⁻¹²·⁶⁷
Solution: (D). pH = -log[H⁺] = -log([10⁻¹⁴]/[OH⁻])
pH SCALE
It was introduced by Sorensen to express the acidic or basic strength of a solution. pH scale is from 0–14 as shown below.
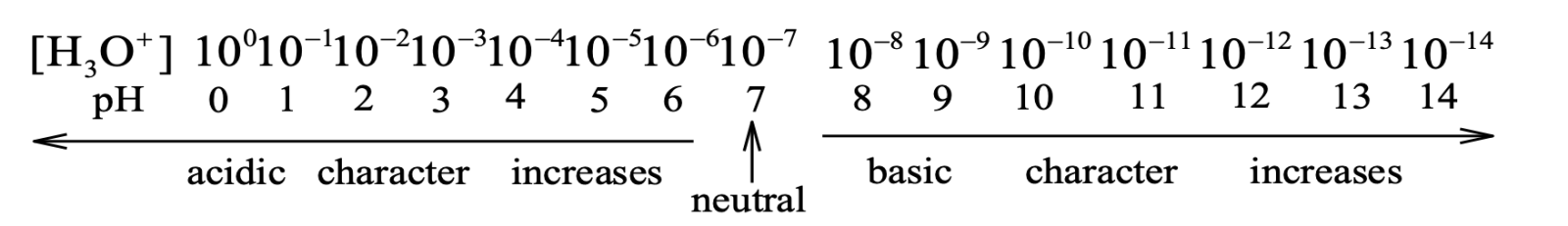
Also pOH = –log [OH⁻]
| Nature of solutions | [H⁺] | [OH⁻] | pH | pOH |
|---|---|---|---|---|
| Acidic solution | > 10⁻⁷ | < 10⁻⁷ | < 7 | > 7 |
| Neutral solution | = 10⁻⁷ | = 10⁻⁷ | = 7 | = 7 |
| Basic solution | < 10⁻⁷ | > 10⁻⁷ | > 7 | < 7 |
where, Ka is the ionisation constant of the weak acid.
pKb = –log Kb
where, Kb it the ionisation constant of the weak base
pKw = –log Kw also pH + pOH = pKw
where, Kw is ionic product of water and pKw = 14 at 25°C
(A) Kw is independent of temperature
(B) At 65°C pH of water is not equal to 7
(C) pKw increases with temperature
(D) pH + pOH = 14, at all temperatures
Solution: (B). pH of water=7 at 25°C. With increase in temperature Kw value increases (i.e. pKw decreases) and pH of water becomes lesser than 7.
1. 2×10⁻⁶ M NaOH
3. 10⁻⁸ M HCl
(A) 1, 2
(B) 2, 3
(C) 3, 4
(D) 2, 3, 4
ACID BASE TITRATIONS USING INDICATORS
Acid–Base indicators are complex–organic molecules which are either weak acids or weak base [Phenolphthalein is a weak acid (HPh) and methyl orange is a weak base (MeOH)] and they change their colour on ionisation, e.g.
(Colourless) (Pink)
As the medium change from acidic to basic and vice–versa, the equilibrium shifts and the colour changes.
Selection of suitable indicators
The indicator used should be such that it shows colour change in the same pH range as required around the equivalence point.
Indicators used in different titrations
| Type of titration | Indicator used |
|---|---|
| Strong acid vs strong base | Phenolphthalein, methyl orange, methyl red |
| Weak acid vs strong base | Phenolphthalein |
| Strong acid vs weak base | Methyl orange and methyl red |
pH–range of some indicators
| Indicator | pH | Acidic colour | Basic colour |
|---|---|---|---|
| Methyl orange | 3.1 – 4.5 | Red | Yellow |
| Methyl red | 4.2 – 6.2 | Pink | Yellow |
| Bromo cresol green | 3.8 – 4.6 | Yellow | Blue |
| Bromothymol blue | 6.0 – 7.5 | Orange | Blue |
| Phenolphthalein | 8.3 – 10.0 | Colourless | Pink |
| Thymol pH | 9.3 – 10.5 | Colourless | Blue |
Universal indicator
It is a mixture of number of indicators which shows colour change over different pH ranges. At the equivalence point pK(indicator) = pH. However, as indicator has a useful colour change over pH range of 2 units. We have, at the equivalence point pH = pK(indicator) ± 1
COMMON ION EFFECT
The degree of dissociation of a weak electrolyte is suppressed by the addition of another electrolyte containing a common ion. This is termed as common ion effect. For example, addition of CH₃COONa in the solution of CH₃COOH suppress the dissociation of CH₃COOH.
SOLUBILITY PRODUCT
For a sparingly soluble salt solubility product is the product of molar concentration of its ions in a saturated solution, e.g.
Initially 1 0 0
At equilibrium 1-s xs ys
Ksp = [A⁺]ˣ [B⁻]ʸ = [xs]ˣ[ys]ʸ = xˣ.yʸ.sˣ⁺ʸ
where, s is the solubility of AxBy in moles/litre.
Ionic product of electrolyte AxBy is also equal to [A⁺]ˣ[B⁻]ʸ but it is applicable to all concentration, may be saturated or unsaturated.
Initially 1 0 0
At equilibrium 1-s s 2s
Ksp = [Pb²⁺][Cl⁻]² = s (2s)² = 4s³
Criteria for precipitation of an electrolyte
- (i) Ionic product > Ksp; Precipitation takes place (super saturated solution)
- (ii) Ionic product = Ksp; The reaction is at equilibrium (saturated solution)
- (iii) Ionic product < Ksp; Number precipitation takes place (Unsaturated solution)
In group II of qualitative analysis H₂S is passed in the acidic solution. Due to common ion effect it decreases the concentration of S²⁻ ion, hence only group II radical (having lower solubility product) are precipitated. In group IV analysis, H₂S is passed in alkaline medium (in NH₄OH) to increase the concentration of S²⁻ so that precipitation of group IV radicals takes place which have higher solubility product.
(A) MA
(B) MB
(C) MC
(D) MD
Solution: (A). Compound having lowest solubility product is precipitated first.
(A) 1
(B) 2
(C) 3
(D) None of these
Solution: (D). Ksp = s × (4s)⁴ = 256s⁵
BUFFER SOLUTION
A solution whose pH does not change by addition of a small amount of acid or base is called buffer solution. Buffer may be of following types
(i) Acidic buffer
It is the mixture of a weak acid and its salt with strong base, e.g. CH₃COONa + CH₃COOH
(ii) Basic buffer
It is the mixture of a weak base and its salt with strong acid, e.g. NH₄OH + NH₄Cl
(iii) Self buffer or single buffer
It is the salt of weak acid and weak base, e.g. CH₃COONH₄
pH of a buffer
or pH = pKa + log[Conjugate base]/[acid]
(ii) Basic buffer, pH = pKb + log[salt]/[base]
or, pH = pKa + log[conjugate acid]/[salt] where, Ka is the ionisation constant of its conjugate acid.
(A) > 7
(B) < 7
(C) = 7
(D) depends on Ka of the acid
Solution: (D). pH = pKa + log[salt]/[acid]
(A) 6.3
(B) 5.5
(C) 6.1
(D) None of these
Solution: (B). pH = pKa + log[salt]/[acid]
5.8 = pKa + log(20/10)
∴ pKa = 5.5
Buffer capacity
It is defined as the number of moles of an acid or base required to be added to one litre of the buffer solution so as to change its pH by one unit.
(A) 30
(B) 0.0307
(C) 2.3
(D) 1
Solution: (B). Buffer capacity = Number of moles of acid or base added in one litre / Change in pH
SALT HYDROLYSIS
It is the process in which a salt reacts with water to give back the acid and base.
or, BA + H₂O ——→ HA + BOH
All salts are strong electrolytes and dissociate completely in aqueous solution.
Degree of hydrolysis
It is defined as the fraction of a total salt which is hydrolyzed.
Hydrolysis constant
or, K[H₂O] = Kh = [HA][BOH] / [BA]
The salts are divided into four categories
(i) Salt of strong acid and strong base
Na⁺ + Cl⁻ + H₂O ——→ Na⁺ + OH⁻ + H⁺ + Cl⁻
or H₂O ——→ H⁺ + OH⁻
Thus, it involves only ionization and no hydrolysis. Thus, in the resulting solution [H⁺]=[OH⁻] and the solution is neutral.
Hence salts of strong acid and strong base do not undergo hydrolysis, e.g. NaCl, NaNO₃, Na₂SO₄, KCl etc.
(ii) Salts of weak acid and strong base
CH₃COONa + H₂O ⇌ CH₃COOH + NaOH
CH₃COO⁻ + H₂O ⇌ CH₃COOH + OH⁻
As it produces OH⁻, the solution is alkaline in nature. Since the anion is undergoing hydrolysis, it is called anionic hydrolysis.
(b) Degree of hydrolysis, h = √(Kh/C) = √(Kw/Ka·C)
(c) pH = -log[H⁺]
pH = ½[log Kw + log Ka + log C]
Examples are, KCN, Na₂CO₃, Na₃PO₄ etc.
(A) 7
(B) < 7
(C) > 7
(D) None of these
Solution: (C). CH₃COONa + H₂O ——→ CH₃COOH + NaOH
The solution will be basic due to presence of strong base.
(iii) Salt of strong acid and weak base
NH₄Cl + H₂O ⇌ NH₄OH + HCl
NH₄⁺ + H₂O ⇌ NH₄OH + H⁺
As it produces H⁺ ions hence, the solution is acidic in nature. Since the cation is hydrolysed it is called cationic hydrolysis.
Degree of hydrolysis, h = √(Kh/C) = √(Kw/Kb·C)
pH = ½[log Kw - log Kb - log C]
(A) NaC₂H₃O₂
(B) NH₄NO₃
(C) CuSO₄
(D) AlCl₃
Solution: (D). AlCl₃ + H₂O ——→ 3HCl + Al(OH)₃
Since more acid is produced hence, more acidic solution is obtained.
(iv) Salt of weak acid and weak base
CH₃COONH₄ + H₂O ⇌ CH₃COOH + NH₄OH
or, CH₃COO⁻ + NH₄⁺ + H₂O ⇌ CH₃COOH + NH₄OH
It involves both cationic and anionic hydrolysis.
The final solution may be neutral, slightly acid or basic depending upon the degrees of ionization of weak acid and weak base produced. In the present case the solution is neutral as the CH₃COOH and NH₄OH are almost equally weak.
Degree of hydrolysis h = √(Kw/(Ka × Kb))
pH = ½[log Kw + log Ka - log Kb]
(A) 0.55×10⁻²
(B) 0.55
(C) 7.63
(D) 1.8×10⁻²
Solution: (B). h = √(Kw/(Ka × Kb)) = √(10⁻¹⁴/(1.8 × 10⁻⁵)²)
Hydrolysis = h × 100 = 0.55%
Solved Examples
(A) 9.30
(B) 7.30
(C) 10.30
(D) 8.30
Sol. (A). pH = pKa + log[KCN]/[HCN] = 9.30
(A) CH₃COONa
(B) NH₄Cl
(C) CH₃COONH₄
(D) NaCl
Sol. (C). It is the salt of weak acid and weak base, hence its degree of hydrolysis will be independent of concentration. h = √(Kw/(Ka × Kb))
(A) pH will decrease while pOH will remain constant
(B) pH will increase while pOH will remain constant
(C) pH will increase while pOH will decrease
(D) Both pH and pOH will decrease
Sol. (A). Kw will increase and hence, pH + pOH will decrease. Molarity of NaOH solution remaining constant, pOH will remain constant but pH will decrease.
(1) Sodium acetate and acetic acid in water
(2) Sodium acetate and hydrochloric acid in water
(3) Ammonia and ammonium chloride in water
(4) Ammonia and sodium hydroxide in water
(A) 1, 3, 4
(B) 1, 3
(C) 1, 2, 4
(D) 2, 4
Sol. (B). Buffer is a solution of a weak acid or base and its salt with strong base or strong acid respectively.
(A) Unionised in the small intestine and in the stomach
(B) Completely ionized in the small intestine and in the stomach.
(C) Ionised in the stomach and almost unionized in the small intestine
(D) Ionised in the small intestine and almost unionsied in the stomach
Sol. (D). Since pH in small intestine is more, the conditions are basic and aspirin will dissociate.
ASSIGNMENT PROBLEMS
(A) NH₄Cl + NH₄OH
(B) CH₃COOH+CH₃COONa
(C) 40 ml of 0.1 M NaCN + 20 ml of 0.1M HCl
(D) All of these
(A) 1.8×10⁻⁹
(B) 1.8×10⁻¹⁶
(C) 1.0×10⁻¹⁴
(D) Can not be calculated
(A) 1 × 10⁻³
(B) 1 × 10⁻⁶
(C) 1 × 10⁻⁸
(D) 1 × 10⁻¹¹
(A) [NH₄⁺][NH₂⁻]
(B) [NH₂⁻][NH₃]
(C) [NH₄⁺][NH₂⁻]
(D) [NH₄⁺]/[NH₂⁻]
(A) 100 ml of M/10 HCl + 100 ml of M/10 NaOH
(B) 55 ml of M/10 HCl + 45 ml of M/10 NaOH
(C) 10 ml of M/10 HCl + 90 ml of M/10 NaOH
(D) 75 ml of M/5 HCl + 25 ml of M/5 NaOH
ANSWERS TO ASSIGNMENT PROBLEMS
4. C
7. C
10. C
13. B