Master S-Block Elements and Ace Your CBSE Chemistry Exam
Stop struggling with confusing textbooks. Get our comprehensive, easy-to-understand s-block notes, packed with solved examples and practice questions. Designed by experts to help you score higher marks with less effort.
A Complete Guide to Every Topic in the S-Block Syllabus
Our notes provide in-depth coverage of all essential concepts. You'll move from basic understanding to complete mastery with clear, structured explanations.
-
Fundamental Properties: Understand the core physical and chemical properties, including electronic configuration, density, ionization energy, and flame colouration for both Group 1 (Alkali Metals) and Group 2 (Alkaline Earth Metals).
-
Key Chemical Reactions: Learn how s-block elements react with water, oxygen, hydrogen, nitrogen, and halogens, with detailed reaction mechanisms and stability trends.
-
Important Compounds: Get detailed explanations on the preparation and properties of crucial compounds like Sodium Carbonate (Washing Soda), Calcium Oxide (Quick Lime), Plaster of Paris, and Bleaching Powder.
-
Hydrogen & Its Compounds: A dedicated section covering Hydrogen's unique position in the periodic table, its isotopes, and the structure and properties of Hydrogen Peroxide (H2O2).
Go Beyond Theory with Proven, Exam-Oriented Learning Tools
These notes aren't just for reading; they are designed to make you practice and perfect your problem-solving skills, ensuring you are fully prepared for any question that comes your way.
-
Learn by Example: Grasp complex concepts quickly with step-by-step solved "Illustrations" that break down the application of theory, such as identifying the strongest reducing agent or understanding polarizing power.
-
Test Your Knowledge: Reinforce your learning at every stage with chapter-end "Exercises" and "Assignment Problems" that mirror the format of CBSE and competitive exam questions.
-
Revise in Minutes: Use the concise "Points to Remember" section for a quick and effective summary of all key facts, trends, and formulas perfect for last-minute revision before the exam.
-
Understand the Exceptions: Confidently tackle tricky questions with special focus on the anomalous behavior of Lithium and Beryllium, which are frequently asked topics.
Ready to Boost Your Chemistry Score?
Don't let s-block elements pull your grade down. Get the focused, high-quality notes you need to excel. Fill out the form to receive a call from our academic counsellor and get a free sample of the notes.
Introduction
s-block consists of group 1 (alkali metals) and group 2 (alkaline earth metals) elements.
The elements are
Group I:
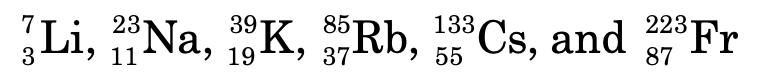
Group I I:
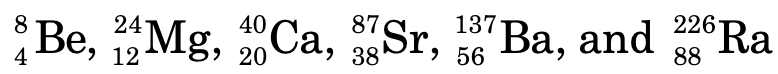
PHYSICAL PROPERTIES
Electronic Configuration
Group I elements contain only one electron in s-orbital (ns1) while group II elements contain two electrons in s-orbital or valence shell (ns2).
Density
Increases down the group as atomic mass increases. In group II, density first deceases from Be to Ca then increases.
Melting and Boiling Points
Melting and boiling points are quite low which decreases further with increase in atomic number due to weakening of metallic bonds.
Structural Geometry
At room temperature, all alkali metals possess body centred cubic structure with co-ordination number 8. However, at low temperature lithium forms a hexagonal close packed structure with co-ordination number 12.
Flame Colouration
In case of s-block elements, the electrons easily get excited to higher energy level by small energy provided by the Bunsen flame due to low ionization energy. These electrons emit energy during de-excitation to ground state. The energy emitted is in the visible region of electromagnetic spectrum which imparts colour to the flame. Moving down the group I.E. decreases hence frequency of emitted light increases. Hence, the colour imparted to the flame shows gradual shift from red to violet on moving down the group.
Group I
|
Element |
Colour |
|
Na |
Golden yellow |
|
K |
Pale violet |
|
Rb |
Purple |
|
Cs |
Sky blue |
Group II
Be and Mg have high I.E., so they do not impart characteristic colour to the flame. However, Ca, Sr and Ba imparts characteristic colour to the flame because of their relative low ionization energies.
|
Element |
Colour |
|
Ca |
Brick red |
|
Sr |
Crimson red |
|
Ba |
Apple green |
|
Ra |
Crimson |
Ionization Energy
Alkali metals have low I.E. and can easily loose their outermost electron, hence shows the following properties.
(i) Photo electric effect
(ii) Electrical conduction: Li+ < Na+ < K+ < Rb+ < Cs+
(iii) Reducing character: Na < Rb < Cs < Li
Example: Which of the following is stronger reducing agent in aqueous solution?
(A) Li
(B) Na
(C) K
(D) Rb
Solution: (A).
Lithium has smallest ionic radius due to this high hydration energy has synergistic effect to lose electron in aqueous solution in respective of high ionization potential of the same.
Hydration of Ions
Alkali metal in aqueous solution exists as M+ (H2O)x. Degree of hydration decreases with ionic size. Hence, Li+ ion is most hydrated. More the hydration, bigger is the size of the hydrated ion. Hence, it has lowest ionic mobility.
Example: Which of the alkali metal has polarizing power close to that of magnesium?
(A) Li
(B) Na
(C) K
(D) Rb
Solution: (A).
Metals having identical charge-size ratio.
Chemical Properties
Alkali metals are quite reactive and readily form ionic compounds. However, lithium can form covalent compounds like alkyl lithium (R – Li) due to its high ionization enthalpy. Chemical reactivity of alkaline earth metals is lower than alkali metals. In case of alkaline earth metals, Be mostly form covalent compounds while Mg compounds are partly covalent and partly ionic while those of Ca, Sr and Ba are purely ionic.
Some chemical properties of alkali and alkaline earth metals are discussed below.
Reaction with Water
In case of alkali metals, electropositive character increases on moving down the group. Hence, reactivity follows the order:
Li < Na < K < Rb < Cs
Similarly, in case of alkaline earth metals, reactivity increases moving down the group. Be does not react with water. While Mg reacts with boiling water. Ca, Sr and Ba react even with cold water.
Group I:
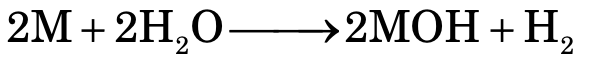
Group II:
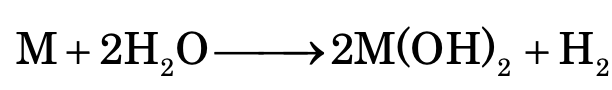
Reaction with Oxygen
Group I:
Affinity for oxygen increases down the group. Thus, Li forms Li2O (lithium oxide) while Na forms peroxide (Na2O2). K, Rb and Cs form super oxide (KO2, RbO2, CsO2). As superoxides have unpaired electron so they are paramagnetic in nature and coloured.
Na2O2 shows yellow colour due to presence of superoxide NaO2 as impurity whereas KO2, RbO2, and CsO2 are orange, brown and orange respectively. Alkali metals are kept under hydrocarbon solution to prevent their reaction with air and moisture.
Group II:
Similarly, for group II elements monoxides and peroxides are formed.
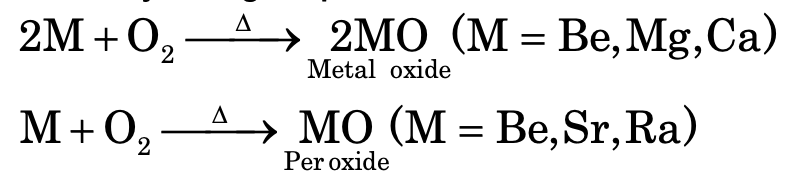
Reaction with Hydrogen
Both group I and group II elements form hydrides of molecular formula, MH and MH2 respectively with hydrogen. Stability of hydrides of group I decreases from LiH to CsH. Be does not react with hydrogen, so BeH2 is obtained by reducing BeCl2 with LiAlH4. CaH2 is known as hydrolith. All hydrides in water evolve hydrogen gas and act as reducing agent.
Reaction with Nitrogen
In group I, only Li reacts with nitrogen to form lithium nitride (Li3N).
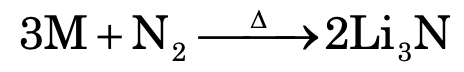
While all group II elements form nitrides with N2, which evolve NH3 gas with water.
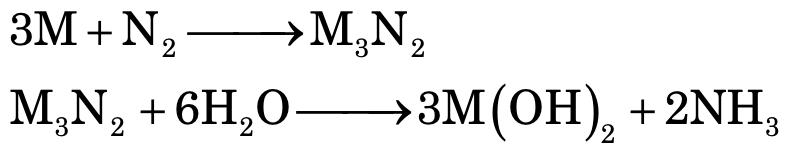
Reaction with Halogen
All group I and group II elements form halides of molecular formula, MX and MX2 respectively. Reactivity of group I elements towards a particular halogen is in the order:
Li < Na < K < Rb < Cs.
While for a particular metal reactivity of halogen is
F2 > Cl2 > Br2 > I2
LiF is ionic while all other lithium halides have covalent character (Fajan’s rule). Hence, they are soluble in organic solvents. All halides of group II elements are hygroscopic in nature and their solubility decreases in the order: MgX2 > CaX2 > SrX2 > BaX2
On descending down the group, the size of atom increases so lattice energy and hydration energy both decreases. But hydration energy decrease more rapidly. Hence, solubility goes on decreasing. However, with fluorides reverse happens.
Reaction with Non-metals
Alkali metals react with sulphate and phosphorus on heating to form disulphide and phosphides.
M + S8 → M2S (Sulphide)
M + P4 → M2P (Phosphide)
Except Be, all other group II elements form carbides of general formula MC2. Be forms BeC2 called methanide.
Strength of Hydroxides
Group I element hydroxides are strong bases and their strength increases from LiOH to CsOH due to decreasing bond energy between metal and oxygen.
Similarly, for alkaline earth metal hydroxides, Be(OH)2 is amphoteric while from Be to Ba basic strength increases.
Solubility Trends
1. Solubility in liquid NH3: All alkali and alkaline earth metals dissolve in liquid ammonia. Alkali metal gives deep blue solution due to presence of ammoniated (solvated) electrons in solution.
M + (x+y)NH3 → M+(NH3)x + e-(NH3)y
These electrons absorb photons of energy falling in red region of spectrum hence appear blue. Similarly, alkaline earth metals are soluble in liquid NH3. In dilute solution, they give blue colour while in concentrated solution they impart bronze colour due to the formation of metal clusters.
2. Solubility of s-block element hydroxides increases going down the group since lattice energy decreases more rapidly than the hydration energy.
Solubility of sulphate decreases in the order:
BeSO4 > MgSO4 > CaSO4 > SrSO4 > BaSO4 for group II elements.
As the size of cations increases, heat of hydration decreases while lattice energy remains almost same (due to large size of sulphate). For group I element sulphates, Li2SO4 is insoluble while others are soluble.
3. Similar is the case with carbonates. Hence, trend is
BeCO3 > MgCO3 > CaCO3 > SrCO3 > BaCO3
Extremely low solubility of alkaline earth metal carbonates in water is used in the precipitation of Ba2+, Sr2+ and Ca2+ as their carbonates in Vth group of qualitative analysis of basic radicals.
Solubilities of carbonates and bicarbonates increases as we move down the group due to lower lattice energies. Thus, order is LiHCO3 < NaHCO3 < KHCO3 < RbHCO3 < CsHCO3
THERMAL STABILITY
Alkali Carbonates and Bicarbonates
Li2CO3 is much less stable and decomposes on strong heating to give Li2O and CO2
Li2CO3 ——Red Hot——> Li2O + CO2
Order for stability is:
Li2CO3 < Na2CO3 < K2CO3 < Rb2CO3 < Cs2CO3
All bicarbonates on heating form carbonates with the evolution of CO2, e.g.
2NaHCO3 ——Δ——> Na2CO3 + CO2 + H2O
LiHCO3 does not exist as a solid but exists in solution.
Alkaline earth metal carbonates decompose on heating giving metal oxide and CO2.
The order is:
BeCO3 < MgCO3 < CaCO3 < SrCO3 < BaCO3
Thermal Stabilities of Sulphates and Nitrates
LiNO3 decomposes to give NO2 and O2 while nitrates of all other alkali metals decomposes on heating to form nitrites and O2.
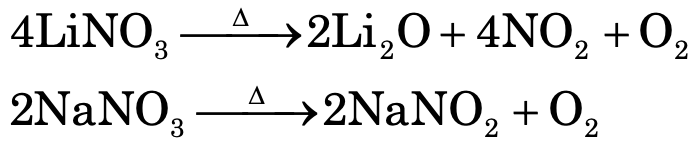
Thermal stability of sulphates increases down the group, i.e.
BeSO4 < MgSO4 < CaSO4 < SrSO4
Anomalous behaviour: Lithium in case of alkali metals and beryllium in alkaline earth metals show anomalous behaviour due to (i) higher electronegativity and (ii) smaller atomic and ionic radii.
COMPOUNDS OF s-BLOCK ELEMENTS
Sodium Carbonate (Na2CO3)
This is prepared by Solvay’s process. Excess of CO2 is bubbled through nearly saturated brine solution containing ammonia. Sodium bicarbonate formed is reacted with sodium chloride. Sodium bicarbonate is filtered off and calcined to give sodium carbonate and CO2. This is “Solvay’s process”.
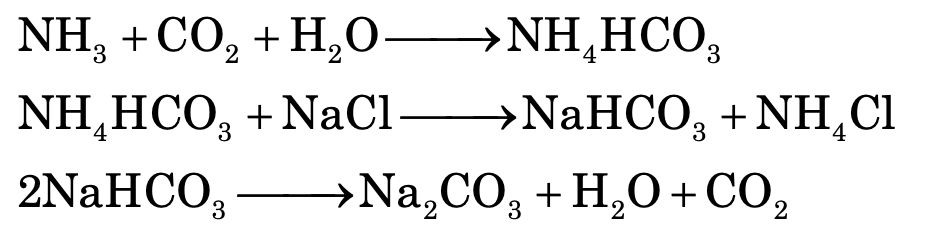
Properties
(i) Aqueous solution absorbs CO₂ yielding sparingly soluble sodium bicarbonate.
Na₂CO₃ + H₂CO₃ ⟶ NaHCO₃
(ii) With acids, it liberates CO₂ and with lime it produces caustic soda (NaOH).
Na₂CO₃ + 2HCl ⟶ 2NaCl + H₂O + CO₂
Na₂CO₃ + Ca(OH)₂ ⟶ 2NaOH + CaCO₃
(iii) Sodium carbonate forms sodium silicate with silica.
Na₂CO₃ + SiO₂ ⟶ Na₂SiO₃ + CO₂
Sodium Bicarbonate (NaHCO₃)
It is sparingly soluble in water and on heating it is converted to anhydrous sodium carbonate. On the reverse, it can be prepared from sodium carbonate as follows.
Na₂CO₃ + CO₂ + H₂O ⟶ 2NaHCO₃
Sodium Sulphate (Na₂SO₄)
Anhydrous salts prepared from sodium chloride.
NaCl + H₂SO₄ ⟶ NaHSO₄ + HCl ↑
NaCl + NaHSO₄ ⟶ Na₂SO₄ + HCl ↑
Hydrated salt Na₂SO₄·10H₂O is called Glauber’s salt and is prepared from anhydrous salt by crystallization from water below 32°C. It is a water soluble crystalline salt which forms large monoclinic prism type crystals.
Calcium Oxide or Quick Lime (CaO)
It is made by decomposing limestone at high temperature of about 1000ºC.
Reaction: CaCO3 →1000ºC CaO + CO2
It is a white amorphous powder, which emits intense white light when heated in oxy-hydrogen flame.
It reacts with silica to form easily fusible calcium silicate.
Reaction: CaO + SiO2 → CaSiO3
With water it evolves high amount of heat and produces slaked lime.
Reaction: CaO + H2O → Ca(OH)2
Calcium Carbonate (CaCO3)
In nature it occurs as limestone, marble, coral, chalk, etc. The white powder is prepared by dissolving limestone or marble in hydrochloric acid and removing iron and aluminum present by precipitating with NH3 and then adding ammonium carbonate to the solution. The precipitate is filtered and dried.
Reaction: CaCl2 + (NH4)2CO3 → CaCO3 + 2NH4Cl
It dissolves in water forming bicarbonate.
CaCO3 + H2O + CO2 ⟶ Ca(HCO3)2
Bleaching Powder (CaOCl2)
Preparation
It is prepared by passing chlorine over slaked lime.
3Ca(OH)2 + 2Cl2 ⟶ CaOCl2 + CaCl2·Ca(OH)2·H2O
Bleaching powder
Properties
- It reacts with dilute acids to give chlorine known as available chlorine.
CaOCl2 + 2HCl ⟶ CaCl2 + H2O + Cl2 ↑
CaOCl2 + H2SO4 ⟶ CaSO4 + H2O + Cl2 ↑ - With water it decomposes into calcium chloride and calcium hypochlorite.
CaOCl2 + H2O ⟶ CaCl2 + Ca(OCl)2 + H2O - It reacts with CO2 from atmosphere and evolves Cl2 which acts as a bleaching agent and oxidizing agent.
CaOCl2 + CO2 ⟶ CaCO3 + Cl2 - On heating bleaching powder, it gives a mixture of chlorate and chloride.
6CaOCl2 ⟶Δ⟶ Ca(ClO3)2 + 5CaCl2
Sodium Carbonate or Washing Soda (Na2CO3·10H2O)
Ammonia – soda process (Solvay process):
When carbon dioxide is bubbled through a brine solution saturated with ammonia it results in the formation of sodium hydrogen carbonate.
NH3 + H2O + CO2 ⟶ NH4HCO3
NaCl + NH4HCO3 ⟶ NaHCO3 ↓ + NH4Cl
2NaHCO3 ———(ignited)——→ Na2CO3 + CO2 + H2O
The filtrate after removal of NaHCO3 contains ammonium salts such as NH4HCO3 and NH4Cl.
NH4HCO3 —Δ→ NH3 + H2O + CO2 2NH4Cl + Ca(OH)2 —Δ→ 2NH3 + 2H2O + CaCl2
Recovery of ammonia
Note: Solvay’s process cannot be used for the manufacture of K2CO3 because potassium bicarbonate is soluble in water.
Sodium Hydroxide (NaOH)
It is prepared by the following process.
Caustic process (Gossage process):
In this process a 10 – 22% solution of sodium carbonate is reacted with calcium hydroxide.
Na2CO3 + Ca(OH)2 ⟶ CaCO3 ↓ + 2NaOH
Electrolytic process:
This process involves electrolysis of an aqueous solution of NaCl.
At anode: 2Cl− ⟶ Cl2 + 2e−
At cathode: 2Na+ + 2e− ⟶ 2Na
2Na + 2H2O ⟶ 2NaOH + H2
Since, chlorine reacts with NaOH even in cold conditions, therefore, electrolysis is carried out in specially designed electrolytic cell, which avoids contact between Cl2 and NaOH. The two such cells are:
- Nelson cell
- Castner–Kellner cell
Magnesium Sulphate or Epsom salt (MgSO4·7H2O)
Magnesium sulphate can be prepared from magnesite (MgCO3) or from kieserite (MgSO4·H2O).
MgCO3 + H2SO4 ⟶ MgSO4 + CO2 + H2O
The resulting solution on concentration and cooling gives crystals of MgSO4·7H2O. MgSO4·7H2O is efflorescent, i.e. it loses water of crystallization on exposure to air.
Plaster of Paris (Hemihydrate of Calcium Sulphates)
It is prepared by heating gypsum (CaSO4·2H2O) to 390 K.
2CaSO4·2H2O ⟶390 K (CaSO4)2·H2O + 3H2O
The temperature should not be allowed to rise above 390 K because above this temperature, the water of crystallization is lost. The resulting anhydrous CaSO4 is called dead burnt plaster because it loses the property of setting with water.
ANOMALOUS BEHAVIOUR OF LITHIUM
Amongst the alkali metals, Li has the smallest size and hence the highest polarizing power. Due to this reason, lithium is different from rest of its family members.
(i) Lithium is harder than any other alkali metal.
(ii) Lithium combines with O2 to form lithium monoxide whereas other alkali metals form peroxides and superoxides.
(iii) Lithium hydride is most stable than the other alkali metal hydrides.
(iv) Lithium carbonate decomposes on heating to evolve CO2 whereas other alkali metal carbonates do not.
(v) Lithium when heated with ammonia forms lithium imide (Li2NH) while other alkali metals form amides of the general formula, MNH2.
ANOMALOUS BEHAVIOUR OF BERYLLIUM
Beryllium is anomalous in many of its properties and shows a diagonal relationship to aluminium in group – 3
- Be is very small and has a high charge density so by Fajans’ rules it should have a strong tendency to covalence. Thus the melting point of its compounds is lower (BeF2 m.pt. 800°C, rest of group about 1300°C) and they are all soluble in organic solvents and hydrolyses in water rather like the compounds of aluminium.
- Be forms many complexes – not typical of Group – 1 and 2.
- Be like Al is rendered passive by nitric acid.
- Be is amphoteric, liberating H2 with NaOH and forming beryllates. Al forms aluminates.
- Be(OH)2 like Al(OH)3 is amphoteric.
- The salts are extensively hydrolyzed.
- The halides are polymeric, with multicentre bonding associated with electron deficiency. BeCl2 usually forms chains but also exists as the dimmer, whilst AlCl3 is dimeric.
- The hydrides and alkyls are also polymeric.
- Be salts are among the most soluble known.
- Be2C, like Al4C3, yields methane of hydrolysis.
HYDROGEN
Hydrogen is the first element in the periodic table and is also the lightest element known. Its atomic form exists only at high temperatures. In the normal element form, it exists as a diatomic molecule, i.e..
Position of Hydrogen in the Periodic Table
Hydrogen, the first element in the periodic table, has the simplest atomic structure of all the elements, and consists of a nucleus of charge + 1 and one orbital electron. The alkali metals also have a single outer orbital electron, but they tend to lose this electron in reactions and form positive ions; by contrast, hydrogen has little tendency to lose this electron but a great tendency to pair the electron and form a covalent bond. The halogens 17, like hydrogen, are one electron short of an inert gas structure. In many reactions the halogens gain an electron and so form negative ions, although hydrogen can only do this in reactions with highly electropositive metals. This behaviour is explained by the atomic structure, the extremely small size of hydrogen atoms and the low electronegativity value. These unique properties make it difficult to place hydrogen in the periodic table, since its properties differ from those of both Group 1 and group 17 elements, and although it is included in both groups in the tables in this book, it could well be put in a group on its own.
Isotopes of Hydrogen
It has been found by mass spectrograph that hydrogen has three isotopes namely; protium, deuterium and tritium. The relative abundance of three isotopes of hydrogen is as under
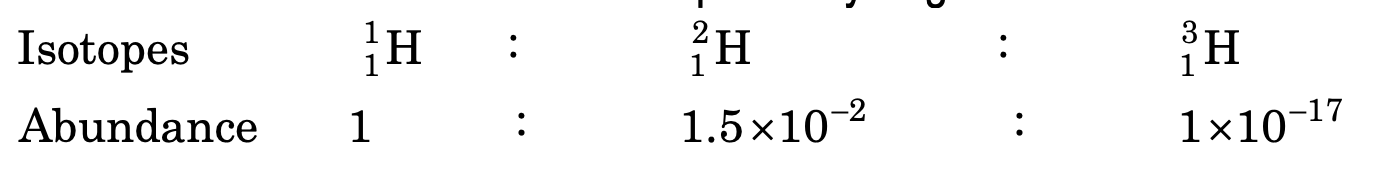
(a) Protium or hydrogen
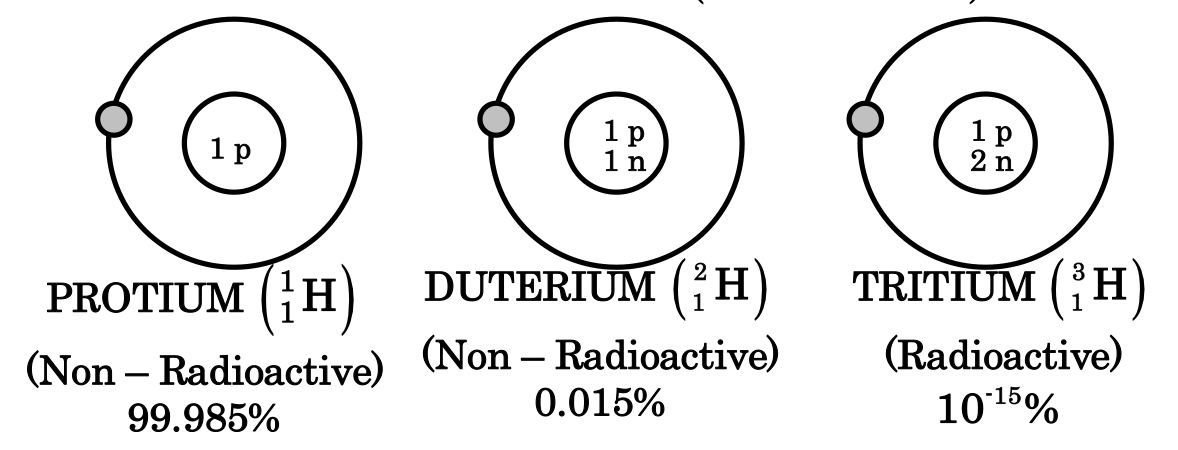
It is represented by the symbol D or 12H. Its atomic number is 1 and mass number is also 1. It has one proton (but no neutron) in its nucleus and one electron in its 1s orbital. Naturally occurring hydrogen contains 99.985% of this isotopes.
(b) Deuterium or heavy hydrogen
It is represented by the symbol D or. It’s atomic number is 1 and mass number is 2. It has one proton and one neutron in its nucleus and one electron in its 1s orbital. Naturally occurring hydrogen has 0.015% of this isotopes mostly in the form of HD.
(c) Tritium
It is represented by the symbol T or 13H. Its nucleus has one proton and 2 neutron and there is one electron in its 1s orbital. It is an extremely rare isotope. Out of 1017 molecules of ordinary hydrogen there is just one molecule of tritium. This isotope of hydrogen is radioactive in nature and emits low energy - particles (t1/2 = 12.33y)
It may be noted that three isotopes of hydrogen have similar chemical properties because of the same electronic configuration,1s1. However due to different mass numbers they have different rates of chemical reactions. For example, reaction between protium and chlorine is 13.4 times faster than that between deuterium and chlorine. Similarly electrolysis of ordinary water (H2O) occurs more rapidly than of heavy water (D2O).Difference in properties arising due to the difference in mass number is referred to as isotopic effect.
(d) Ortho and para hydrogen
When the spins of the nuclei are in the same direction (parallel spins), dihydrogen is called ortho hydrogen and when the spins are in the opposite direction (anti parallel spins), dihydrogen is called para hydrogen.
Example: The property of hydrogen which distinguishes it from other alkali metals is
(A) its electropositive character
(B) its affinity for non-metals
(C) its reducing character
(D) its non-metallic character
Solution: (D).
When fused NaH is electrolyte hydrogen deposited on anode while sodium on
cathode.
HYDRIDES
Dihydrogen combines with a number of elements to form binary compounds called hydrides. Their general formula being where M represents the element and x the number of hydrogen atoms.
Depending upon the physical and chemical properties, the hydrides have been divided into the following three broad categories:
- Ionic or salt-like or saline hydrides
- Metallic or Interstitial hydrides
- Molecules or Covalent hydrides
Saline hydrides or ionic hydrides
These are binary compounds of hydrogen and elements which are more electropositive than hydrogen such as alkali metals, alkaline earth metals (except Be), etc. Saline hydrides are formed by the transference of electron from metal to hydrogen. Some common examples of this category are: etc.
Covalent hydrides or molecular hydrides
These are binary compounds of hydrogen and elements of comparatively high electronegativity such as p- block elements. In these hydrides, H atoms are bonded to the other atoms by covalent bonds. Some examples of covalent hydrides are, HCl, H2O, PH3, NH3 etc.
Interstitial hydride or metallic hydrides
These are binary compounds of hydrogen and transition elements.
These hydrides are generally formed by the
(a) transition metals of group 3, 4, 5 of d- block;
(b) Cr metal of group 6 and
(c) f – block elements.
It may be noted that elements of group 7, 8, 9 of d – block do not form hydrides at all. This inability of metal, of group 7, 8, 9 of periodic table to form hydrides is referred to as hydride gap of d – block.
In these compounds H atoms are supposed to occupy interstitial position in the metal lattices. Some scientists consider these compounds as simply solid solutions of hydrogen. The composition of these hydrides may not correspond to simple whole number ratio and therefore, they are also called non-stoichimotric hydrides. Their composition is also found to vary with the conditions of temperature and pressure. Some examples of interestial hydrides of elements of group 3 to 5 are ScH2, YH2, YH3, LiH3, CrH, TiH2, ZrH2, HfH2, VH, NbH, NbH2, TaH etc.
HYDROGEN PEROXIDE (H2O2)
Preparation
(i) Lab Method: It is prepared by the action of cold, dilute sulphuric acid on sodium or barium peroxide.
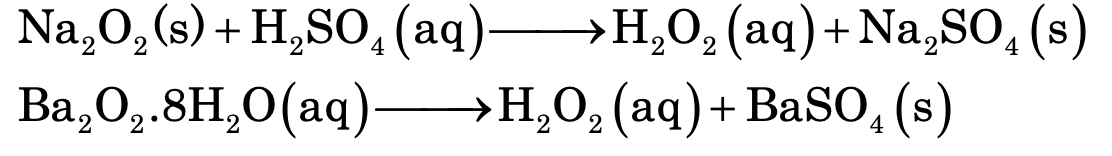
Anhydrous barium oxide is not used because the precipitated BaSO4 forms a protective layer on the unreacted barium peroxide and thus prevent its further participation in the reaction. However, it can be overcome by using phosphoric acid.
(ii) By Electrolysis: It can also be prepared by the hydrolysis of peroxydisulphuric acid which is obtained by the electrolytic oxidation of sulphuric acid.
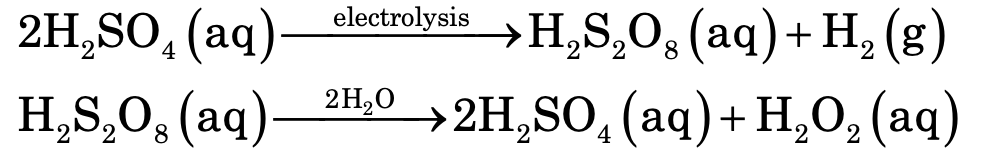
(iii) It can be prepared by auto-oxidation of 2-ethyl anthraquinol. The net reaction is a catalytic union of H2 and O2 to yield hydrogen peroxide.
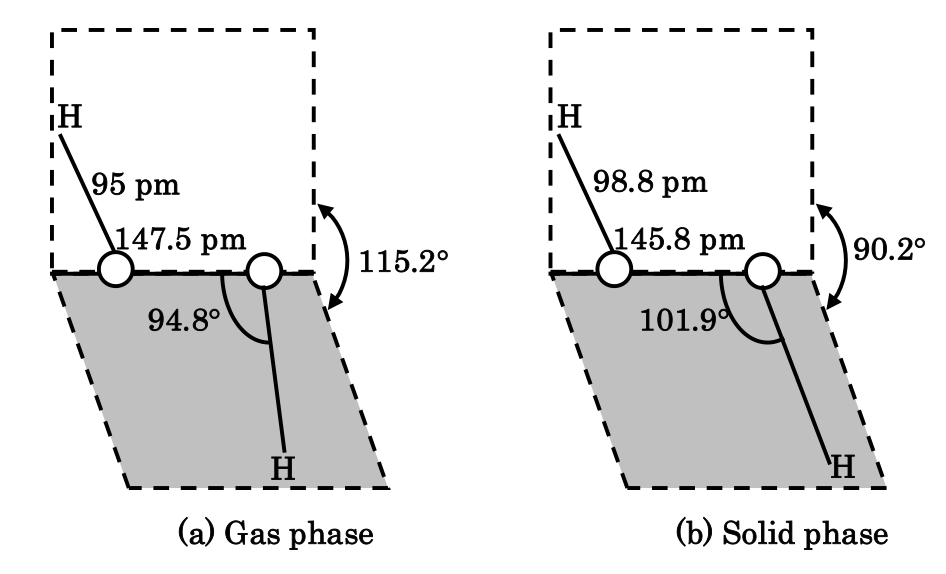
Structure of hydrogen peroxide
As established by X-ray studies, hydrogen peroxide molecules has a non-planar structure. The molecules dimensions in gas phase and that in solid phase have given in figure. (a) and (b) respectively. In the crystal, the dihedral angle (111.5o) reduces to on account of hydrogen bonding. The two oxygen atoms are joined by a single electron-pair bond.
The O–O Linkage is also called peroxide linkage.
Chemical properties
(a) Decomposition
It is a unstable liquid readily decomposes on heating or on long standing to give water and dioxygen. It is an example of disproportionation decomposition is suppressed by addition of glycerol, acetanilide or phosphoric acid.
(b) Acidic behaviour
Pure hydrogen peroxide is a weak acid (at 298 K).
It ionizes in water as:
H2O2 → H+ + H2O- (hydroperoxide)
HO2- → H+ + O22- (peroxide ion)
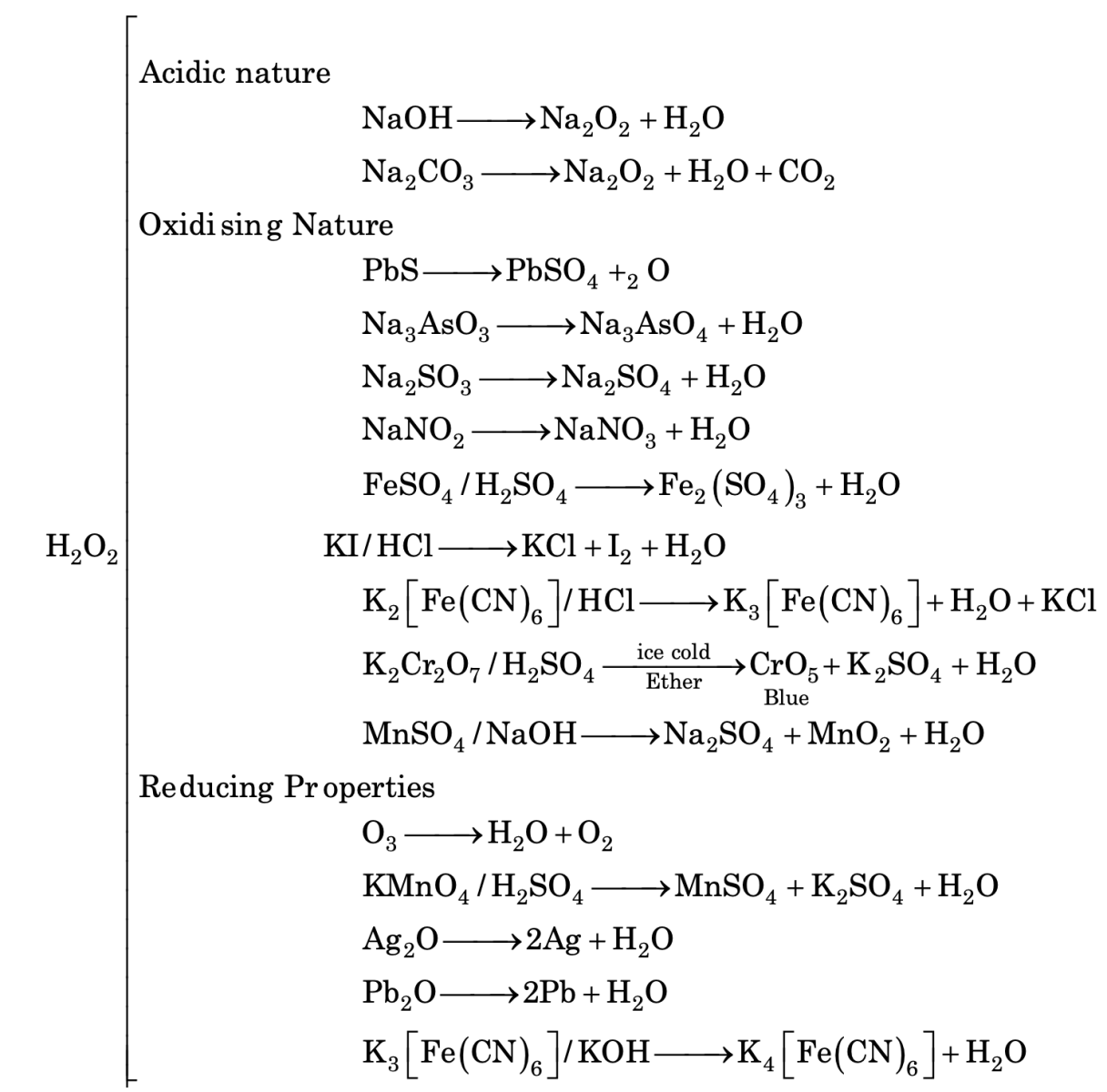
Its acidic character can be shown its ability to neutralize bases such as NaOH, Na2CO3 etc, to form corresponding peroxides.
2NaOH + H2O2 ⟶ Na2O2 + 2H2O
2Na2CO3 + H2O2 ⟶ Na2O2 + H2O + CO2
Hydrogen peroxide has an interesting chemistry because of its ability to act as oxidizing as well as reducing agent both in acidic and basic solutions.
OXIDIZING NATURE OF H2O2
H2O2 can act as oxidizing agents in acidic as well as basic medium as described below.
In acidic medium
H2O2 + 2H+ + 2e- ⟶ 2H2O
In basic medium
H2O2 + 2e- ⟶ 2OH-
Bleaching action
The bleaching action of hydrogen peroxide is due to the nascent oxygen which it liberates on decomposition.
H2O2 ⟶ H2O + [O]
The nascent oxygen combines with colouring matter which, in turn, gets oxidised. Thus, the bleaching action of H2O2 is due to the oxidation of colouring matter by nascent oxygen. It is used for the bleaching of delicate materials like ivory, feather silk, wool etc.
Colouring matter + [O] ⟶ Colourless matter + [O]
Addition reactions
Hydrogen peroxide reacts with alkenes to form glycols.
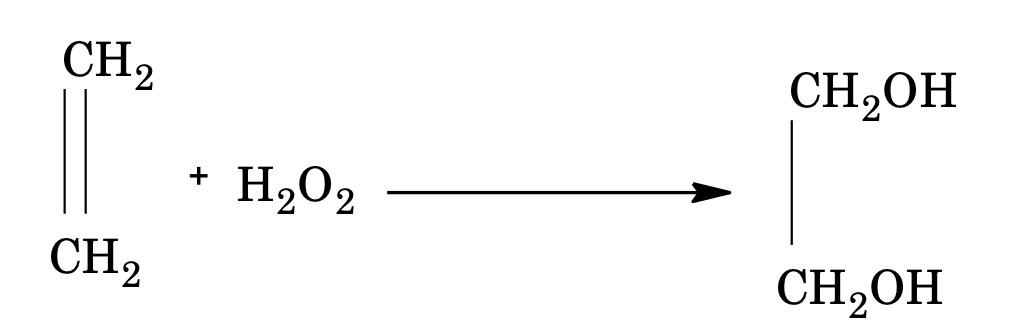
Strength of H2O2 solution:
Strength of H2O2 is expressed in terms of volume of oxygen at NTP that one volume of hydrogen peroxide gives on heating.
The commercial sample are marked as 10 volume, 15 volume etc. 10 volume means that one volume of hydrogen peroxide gives 10 volume of oxygen at NTP.
Let, the volume strength is “V”.
2H2O2 → 2H2O + O2
2(2 + 32) = 68 g 22400 ml at NTP
Then at NTP, “V” litre oxygen will be given by 1 litre of H2O2.
g/litre strength of H2O2 = (68 / 22.4) × V
N = strength / EM = (68 / 22.4) × (V / 17) = V / 5.6
i.e. volume strength = 5.6 × normality
Similarly, volume strength = 11.2 × molarity
Points to Remember
1. On moving down the group from Li to Cs, electropositivity, atomic radii, atomic volume, reactivity, reducing power, conductivity, solubility of salts with small anion and density show increasing trend.
2. Stability and solubility orders of carbonates, nitrates of alkali metals and bicarbonates are:
Li2CO3 < Na2CO3 < K2CO3 < Rb2CO3 < Cs2CO3
Li2CO3 < NaNO3 < KNO3 < RbNO3 < CsNO3
LiHCO3 < NaHCO3 < KHCO3 < RbHCO3 < CsHCO3
3. Stability of alkali metal peroxides and superoxides are in the order:
N2O2 < K2O2 < Rb2O2 < Cs2O2
NaO2 < KO2 < RbO2 < CsO2
4. Solubility, stability and basic strength of alkali and alkaline earth metal hydroxides increases in the following order:
LiOH < NaOH < RbOH < CsOH
Be(OH)2 < Mg(OH)2 < Ca(OH)2 < Sr(OH)2 < Ba(OH)2
5. Degree of hydration of alkali metal ions decreases in the order:
Li+ > Na+ > K+ > Rb+ > Cs+
The relative ionic radii in water also decreases in the same order.
6. Only lithium combines directly with carbon to form lithium carbide, Li2C2. Other alkali metals react with ethylene to form corresponding metal carbides.
7. Magnesium ions are present in chlorophyll- a, which is a green pigment in plants and which absorbs light and is essential for photosynthesis.
8. Aqueous Ba(OH)2 is known as baryta water.
9. Be and Mg crystallize in hcp, Ca and Sr in ccp and Ba in bcc structure.
10. K2CO3 cannot be prepared by Solvay process as KHCO3 is more soluble in water than NaHCO3.
11. Isotopes of hydrogen: Protium (1H), Deuterium (2H or D), Tritium (3H or T).
12. Allotropes of hydrogen: orthohydrogen (o-H2), parahydrogen (p-H2).
13. Molecular forms: H2.2H2 (D2), 3H2 (T2), HD, HT etc.
14. Ortho-hydrogen have parallel nuclear spins and para-hydrogen have antiparallel nuclear spins.
15. Hydrogen under very high pressure is expected to behave like a metal.
16. H2O2 is a colourless some what dense, less volatile liquid than water.
17. H2O2 (gas phase) dihedral angle 111.5o.
18. H2O2 (solid pahse) dihedral angle 110K is 90.2o.
19. H2O2 is weak acid Ka = 1.55 ´ 10-12 at 298 K.
20. H2O2 can used as oxidizing and reducing agent in both medium acidic and basic medium.