Aufbau Principle Rules For Filling Electrons in Orbitals
The Aufbau Principle is a fundamental concept in chemistry that helps scientists and students determine how electrons fill up atomic orbitals in an atom. It serves as a guide for arranging electrons within an atom in order of increasing energy levels, following a specific sequence. Understanding this principle is essential for building electron configurations, which are important for predicting the chemical behavior of elements.
Also Check: Significance of Revision and Method of Revision
What Are Atomic Orbitals?
Before diving into the Aufbau Principle, it’s important to understand atomic orbitals. An orbital is a region around an atom’s nucleus where there is a high probability of finding electrons. Each orbital can hold a maximum of two electrons. There are different types of orbitals—s, p, d, and f—each with distinct shapes and energy levels.
- s-Orbital: Spherical in shape and can hold up to 2 electrons.
- p-Orbital: Dumbbell-shaped and can hold up to 6 electrons (in three sub-levels: px, py, pz).
- d-Orbital: More complex in shape, can hold up to 10 electrons (in five sub-levels).
- f-Orbital: Highly complex in shape, can hold up to 14 electrons (in seven sub-levels).
Also Check: Aditya Birla Scholarship 2024
Understanding Electron Configurations
Electron configuration is the arrangement of electrons in an atom’s orbitals. Electron configurations are important because they give insights into an atom’s stability, reactivity, and bonding tendencies. For example, knowing the electron configuration can help predict how an element will react with others in a chemical reaction.
The Basics of the Aufbau Principle
The term “Aufbau” comes from a German word meaning “building up.” The Aufbau Principle is often called the building-up principle because it states that electrons fill atomic orbitals starting from the lowest energy level to higher energy levels. According to this principle:
- Electrons fill orbitals in a specific order based on their energy.
- Lower-energy orbitals fill before higher-energy orbitals.
- Each orbital can hold a maximum of two electrons.
Following this principle, the electrons in an atom arrange themselves in the most stable configuration, filling lower energy levels first.
Also check: JEE Main 2025 Chapter-Wise Weightage Expected
Rules for Filling Electrons Using the Aufbau Principle
To apply the Aufbau Principle correctly, there are several rules and guidelines that need to be followed:
- Lowest Energy First: Start by filling the orbital with the lowest energy and then proceed to higher energy orbitals. This rule helps determine the order in which electrons are added to orbitals.
- Pauli Exclusion Principle: No two electrons in an atom can have the same set of quantum numbers. In simpler terms, each orbital can hold only two electrons, and they must have opposite spins.
- Hund’s Rule of Maximum Multiplicity: When electrons fill orbitals of the same energy (like the p, d, or f sub-levels), they first fill empty orbitals singly before pairing up. This reduces electron repulsion and stabilizes the atom.
These rules help us predict the order in which electrons will occupy the different atomic orbitals in an atom.
Also Check: Best Self-study Plan For Class 12th Board Exams
Order of Orbital Filling Based on Energy Levels
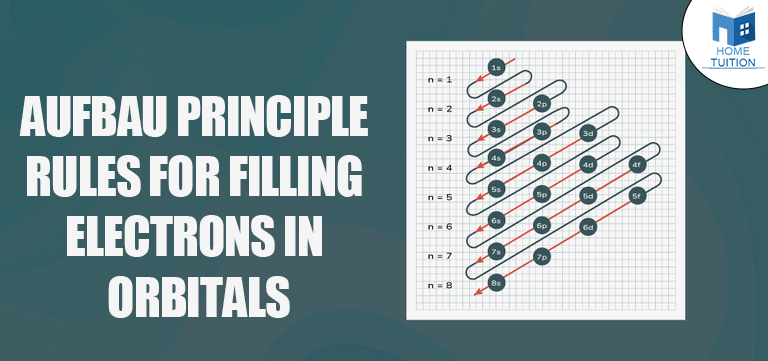
The energy levels follow a specific order, which is not strictly sequential (like 1, 2, 3, etc.). Instead, the filling order is determined by the (n + l) rule:
- Here, n represents the principal quantum number (indicating the energy level).
- l represents the azimuthal quantum number (indicating the shape and type of orbital).
The orbitals are filled in the following order:
- 1s
- 2s
- 2p
- 3s
- 3p
- 4s
- 3d
- 4p
- 5s
- 4d
- 5p
- 6s
- 4f
- 5d
- 6p
This order may seem confusing initially, but it can be easily visualized using an Aufbau diagram or memorized with practice.
Also Check: Important Formulas for JEE Exam
Why the Aufbau Principle Is Important
The Aufbau Principle provides a framework to predict and understand how electrons are distributed within an atom. This information is crucial because:
- Predicting Chemical Behavior: Knowing the electron configuration of an atom helps in understanding its chemical reactivity and bonding.
- Explaining Periodic Trends: The Aufbau Principle, combined with Hund’s Rule and the Pauli Exclusion Principle, explains many periodic trends in the periodic table, like atomic size, ionization energy, and electronegativity.
- Determining Stability: Elements achieve greater stability by reaching a complete or half-filled electron configuration, which is why certain electron configurations (like noble gases) are more stable.
Also Check: How To Plan Your Study Time Table for Class 12
Limitations of the Aufbau Principle
While the Aufbau Principle is highly useful, it does have some limitations:
- Electron-Electron Repulsion: The principle assumes that electrons are added sequentially without considering the effects of electron repulsion in multi-electron atoms.
- Anomalous Configurations: Some elements, especially transition metals like chromium (Cr) and copper (Cu), have configurations that don’t strictly follow the Aufbau order due to added stability from half-filled or fully-filled orbitals.
- Complexity in Larger Atoms: For atoms with many electrons, the filling order can become less predictable due to the interactions between electrons in different shells and sub-levels.
Despite these limitations, the Aufbau Principle remains a helpful rule of thumb for understanding electron configurations in most cases.
Examples of Electron Configurations Using the Aufbau Principle
Let’s apply the Aufbau Principle to write the electron configurations of a few elements:
1. Hydrogen (H)
- Atomic number = 1
- Configuration: 1s¹
Since hydrogen has only one electron, it fills the 1s orbital first.
2. Helium (He)
- Atomic number = 2
- Configuration: 1s²
The two electrons of helium fill the 1s orbital, making it complete.
3. Oxygen (O)
- Atomic number = 8
- Configuration: 1s² 2s² 2p⁴
Oxygen has eight electrons. Following the Aufbau order:
- The first two electrons go into the 1s orbital.
- The next two electrons go into the 2s orbital.
- The remaining four electrons fill the 2p orbital, resulting in a 2p⁴ configuration.
4. Magnesium (Mg)
- Atomic number = 12
- Configuration: 1s² 2s² 2p⁶ 3s²
Magnesium’s 12 electrons are distributed as follows:
- Two electrons in 1s, two in 2s, six in 2p, and the last two in the 3s orbital.
5. Iron (Fe)
- Atomic number = 26
- Configuration: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁶
For iron, after filling lower-energy orbitals, we reach the 3d orbital, where six electrons are added according to the order.
Conclusion
The Aufbau Principle is a foundational rule in chemistry that explains how electrons are arranged in an atom’s orbitals. By following the principle's guidelines—filling lower-energy orbitals first and applying Hund’s Rule and the Pauli Exclusion Principle—we can predict the electron configurations of atoms. While there are a few exceptions and limitations, the Aufbau Principle offers a reliable method for understanding the structure of atoms and their chemical behavior. For students and chemistry enthusiasts, mastering this principle is key to exploring more complex concepts in atomic structure and chemical reactions.
Using this knowledge, you can now confidently write electron configurations and deepen your understanding of how atoms form bonds and interact with each other, which is essential in the study of chemistry.
FAQs
The Aufbau Principle, also known as the "building-up principle," is a rule in chemistry that states electrons fill atomic orbitals in order of increasing energy levels, starting from the lowest energy orbital to higher energy orbitals. This principle helps predict how electrons are arranged in an atom’s electron configuration.
The Aufbau Principle is essential because it helps us understand and predict the arrangement of electrons in atoms, which influences the chemical properties, stability, and reactivity of elements. Electron configurations derived from this principle explain trends across the periodic table and help predict bonding behaviors.
Atomic orbitals are regions around an atom’s nucleus where there is a high probability of finding electrons. They come in different shapes and types, including s, p, d, and f orbitals. Each orbital can hold a specific number of electrons: s-orbitals hold 2, p-orbitals hold 6, d-orbitals hold 10, and f-orbitals hold 14.
Electrons fill orbitals starting from the lowest energy level and progress to higher ones. The order is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, and so on. This sequence is based on the energy levels of each orbital, which follow the (n + l) rule—where lower (n + l) values are filled first.
The Aufbau Principle involves three main rules:
- Lowest Energy First: Electrons fill the lowest energy orbital available.
- Pauli Exclusion Principle: No two electrons can have the same set of four quantum numbers, meaning each orbital can hold only two electrons with opposite spins.
- Hund’s Rule: Electrons will occupy empty orbitals of the same energy singly before pairing up, minimizing electron repulsion.
The (n + l) rule is a guideline used to determine the order in which orbitals fill with electrons. The principal quantum number (n) indicates the energy level, and the azimuthal quantum number (l) indicates the orbital type. Lower (n + l) values are filled first, and in cases of equal (n + l) values, the orbital with the lower n value is filled first.
Yes, let’s consider the element oxygen (O) with an atomic number of 8:
- The first two electrons fill the 1s orbital: 1s²
- The next two electrons fill the 2s orbital: 2s²
- The remaining four electrons fill the 2p orbital: 2p⁴
The electron configuration of oxygen is 1s² 2s² 2p⁴.
Yes, some elements, especially transition metals like chromium (Cr) and copper (Cu), have configurations that don’t follow the Aufbau order strictly. This is because half-filled or fully-filled d-orbitals offer extra stability, resulting in slightly different electron configurations.
The Aufbau Principle, along with Hund’s Rule and the Pauli Exclusion Principle, helps explain periodic trends such as atomic size, ionization energy, and electron affinity. Electron configurations determine an element's position on the periodic table, influencing its chemical reactivity and bonding preferences.
While highly useful, the Aufbau Principle has limitations:
- It assumes electrons fill orbitals sequentially without considering electron-electron repulsion in larger atoms.
- Certain transition and heavier elements deviate from the expected electron configurations.
- The filling order becomes complex in atoms with many electrons due to interactions between electrons in different shells and orbitals.
A helpful way to remember the filling order is by using an Aufbau diagram or memorizing the sequence: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s. Visual aids like diagrams and practice can also make it easier to remember this sequence.
The Aufbau Principle applies broadly, especially for elements with fewer electrons. However, it may not perfectly describe electron configurations for all elements, particularly some transition metals and heavier elements where electron-electron interactions can lead to deviations
Recent Blogs
- What is Mathematical Olympiads?
- Indian Air Force Group X and Y Exam
- Difference between CSIR NET and UGC NET
- India Post GDS Recruitment 2025
- MAH MBA Exam
- MP TET 2022 Exam
- Top MBA Colleges in India
- MCQ on On the Trail of the Earliest People
- Roles of Tutoring and Tuitions
- Why you need a good Tutor
- Online teaching: The role of parents
- Technology with traditional teaching?
- How to maximize your child’s potential?
- How to reduce stress?
- What makes a good tutor?