Definition of Atom
An atom is electrically neutral, the smallest individual particle of an element which can take part in chemical reactions.
Definition of Molecules
It is the smallest particle of matter which can exist in a free state and is made up of atoms. Molecules having one atom in the gaseous state are called monoatomic, e.g. helium and argon. Molecules having two atoms are called diatomic, e.g. hydrogen gas, oxygen gas, etc.
Daltons Atomic theory
According to this theory:
(i) Every element is made up of atoms.
(ii) An atom can neither be created nor destroyed.
(iii) Atoms of the same element are identical in mass, size and shape.
(iv) Each element is characterised by the weight of its atom.
(v) Atoms react with each other to form compounds in a simple fixed numerical ratio such as 1 : 1, 2 : 1, 1 : 2, 2 : 3 etc.
Cathode Ray Experiment
These are streams of electrons produced in the discharge tube containing gas (which is a poor conductor of electricity) at a very low pressure.
(i) Cathode rays originate from the cathode and flow towards the anode.
(ii) These are negatively charged and travel in a straight line.
(iii) They cast the shadow of the object in their path.
(iv) Deflected by electric and magnetic fields (charged).
(v) The value of e/m (specific charge) of the particles of cathode rays does not depend upon the material of the cathode and the nature of the gas taken in the discharge tube.
(vi) No electric current flows through a vacuum or the discharge tube containing gas at one atmosphere.
(vii) They produce a heating effect when struck with an object.
Anode Ray Experiment
These are beams of positively charged particles. For example, H+, He+ and He2+, etc.
(i) Anode rays do not originate from the anode; these are produced in the space between the anode and cathode.
(ii) Charge to mass ratio of the particles (e/m) in anode rays depends on the nature of the gas taken.
(iii) Deflection of the positive rays by the electric and magnetic field is smaller than cathode rays, indicating that the positive rays have greater mass than cathode rays.
Fundamental Particle of an Atom
Each atom is made up essentially of three fundamental particles: electron, proton and neutron. Some other uncommon fundamental particles are:
Mesons (charge −1, +1 or 0), neutrino (negligible charge), antineutrino (negligible charge), etc.
1. Electron
The electron was discovered by J. J. Thomson (1887) by the study of cathode rays.
(i) An electron is a negatively charged particle having a charge of 1.6 × 10-19coulombs and Mass 9.1 × 10-31 Kg
(ii) The charge on the electron was determined by Millikan’s oil drop experiment.
(iii) The charge-to-mass (e/m) ratio of the electron was determined by Thomson using a spectrometer.
(iv) Charge on one mole of electrons = 96500 C (1 Faraday)
(v) Mass of one mole of electrons = 0.55 mg
(vii)The Van der Waals forces of attraction increase as the number of electrons in a molecule decreases.
2. Proton
Proton was discovered by Goldstein through the study of anode rays. It is a positively charged particle with having charge of 1.6 × 10-19 mass of 1.672 × 10-27 Kg
3. Neutron
Neutron was discovered by Chadwick (1932) by bombarding particles (He2+) on beryllium atoms.
(i) The charge on the neutron is zero, and the mass is 1.674 × 10-27 Kg
(ii) Neutrons and protons are found in the central core of the atom, called the nucleus.
4. Nucleus
The nucleus was discovered as a result of Rutherford’s scattering experiment.
(i) It is the small central core of an atom, having a diameter of approximately 10−15 m. Diameter of atom = 10−11 m.
(ii) The radius of the nucleus is expressed in Fermi (1 Fermi = 10−15 m) and is given by where A is the atomic mass and Ro is the proportionality constant. The value of Ro lies between. The nuclear radii lie in the range 1.5 to 6.5 fermi.
(iii) The force that binds electrons to the nucleus is coulombic.
(iv) The Nucleus is responsible for the entire mass of the atom.
(v) A nucleus is stable when its neutron-proton ratio is near unity.
(vi) Density of nucleus = 1014 g / cm3 or 108 tonnes / cm3
Atomic Terms used in theory
(i) Nuclide: Various species of atoms in general.
(ii) Nucleons: Protons and neutrons are collectively called nucleons.
(iii) Mass number (A): Sum of protons and neutrons.
(iv) Atomic number (z): Number of protons in the nucleus of an atom.
Atomic mass = Total mass of protons (mp) + Total mass of neutrons (mn)
(v) Isobars: Atoms having the same mass number but different atomic number, e.g. 15P32, 16S32
(vi) Isotopes: Atoms having the same atomic number but different atomic masses, e.g. 92U235, 92U238
(vii) Isotones: Atoms having the same number of neutrons but different mass numbers, e.g. 8O16, 6C14,7N15
(viii) Isosteres: Species having the same number of atoms and electrons, e.g. CO2, N2O.
(ix) Isoelectronic species: Atoms, molecules or ions having the same number of electrons, e.g. N2, CO, CN−.
(x) Isodiaphers: Atoms for which the difference between neutrons and protons is the same, e.g. 8O17, 7N15
and
(xi) Nuclear isomers: Atoms with the same atomic and mass number but different radioactive properties,
(xii) Atomic mass unit: It is equal to 1/12th the mass of a carbon atom.
Atomic weight of an element- Weight of one atom of the element divided by 1/12th the weight of one atom of Carbon
It is relative, not absolute.
1 a.m.u. = 1.66 × 10-24 Gm = 1.66 × 10-27 Kg= 931.5 MeV
Introduction to the Atomic Model
Thomson’s Model atom is made up of a positive charge, and the negative charges are embedded in the positive charge. It is also called plum - pudding model. It could not satisfactorily explain the properties of an atom.
Rutherford’s Model
In Rutherford’s scattering experiment, particles from radium or polonium were allowed to hit a thin foil of gold. Most of them passed through without undergoing any deflection (showing the presence of large empty space in the atom). Some were deflected through small angles (showing the presence of a small charged body in the atom). A few were deflected back through the centre (showing that the charge body is small but heavy and present at the centre).
This experiment led to the following conclusions:
(i) An atom is spherical and mostly hollow.
(ii) The whole of the positive charge and mass of the atom is present in the small area in the centre called the nucleus.
(iii) The electrons are revolving outside the nucleus.
(iii) The number of protons in the nucleus is equal to the number of electrons revolving outside the nucleus.
(iv) The centrifugal force required for the circular motion of electrons is provided by the electrostatic attraction between protons and electrons.
Drawbacks of Rutherford’s model of the atom
(i) Could not explain the stability of the atom.
(ii) Could not explain the line spectrum of the hydrogen atom.
MOSELEY EQUATION
It relates the frequency of X-ray to the atomic number of the element, Root V = a(Z-b), where a and b are constants and Z is the atomic number.
ELECTROMAGNETIC WAVE THEORY
According to this theory, electromagnetic radiation is made up of electric and magnetic fields oscillating perpendicularly to each other and the direction of propagation. All electromagnetic radiations travel at the speed of light and does not need any medium for propagation.
IMPORTANT CHARACTERISTICS OF WAVE
A wave propagates in the form of alternate crests and troughs.
(i) Wavelength: It is the distance between two neighbouring crests or troughs.
(ii) Frequency: It is the number of waves passing per second. It is related to wavelength as
V= C/ Wave-length
(iii) Velocit:y It is the distance travelled by a wave in one second.
(iv) Amplitude (A): It is the maximum height of the crest or depth of the trough.
(v) Wave num: It is the number of wavelengths per cm.
ELECTROMAGNETIC SPECTRUM
The arrangement of electromagnetic radiations in order of increasing wavelengths or decreasing frequencies is called the electromagnetic spectrum.
ATOMIC SPECTRUM
(i) Absorption spectrum: Some substances absorb the energy when white light is passed through their solution or vapours, and this results in some dark lines in the continuous spectrum of white light. This is called the absorption spectrum.
(ii) Emission spectrum: The atom loses energy in the form of radiation by the transition of electrons from a higher to a lower energy level. This energy corresponds to the line of a specific wavelength. These lines constitute the emission spectrum.
Types of emission spectra
(a) Continuous spectra: The seven colours in the spectrum of white light are very close and constitute continuous spectra.
(b) Line spectra: The spectrum of a hydrogen atom contains different lines separated by dark bands. This type of spectrum is called a line spectrum.
Every element gives a characteristic line spectrum different from other elements. Hence, it is like the fingerprint of the element.
Line spectrum of H-atom: The Hydrogen spectrum contains different series of lines, which are given in the diagram.
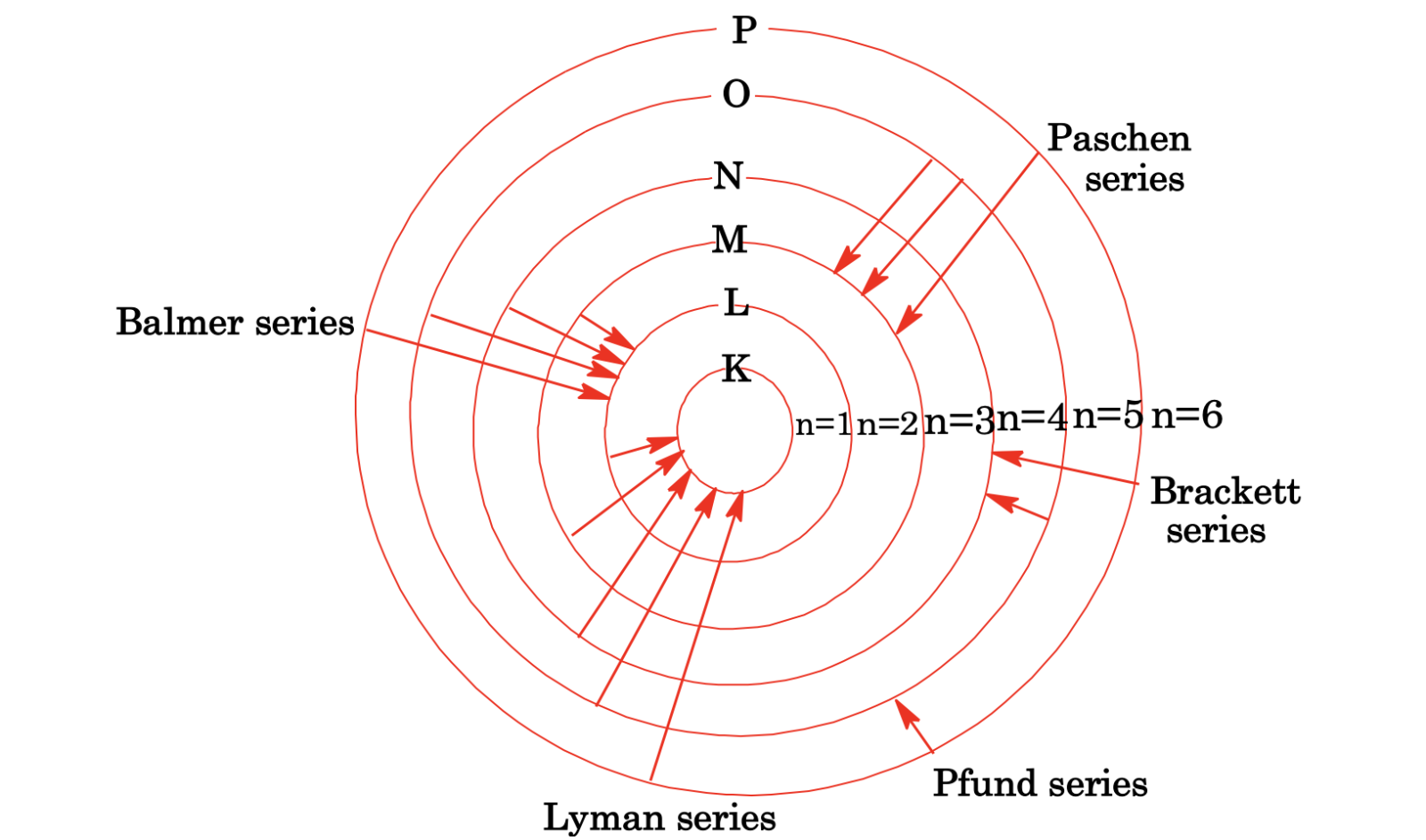
Fig: Different series of H-spectrum
Note:
(i) The intensity of the spectral lines in a particular series decreases as the value of n of the outer shell increases, e.g. in the Lyman series first line (n2 = 2, n1 = 1) has greater intensity than the second line (n2 = 3, n1 = 1).
(ii) As the distance from the nucleus increases, the energy gap between energy levels decreases.
Hydrogen-like species: These have one electron like the hydrogen atom, e.g. He+, Li++, etc.
Number of Spectral Lines
(i) When the final state is the ground state (n1 = 1) and n2 = n
Number of waves= n(n-1)
(ii) When the final state is not the ground state
= (n2- n1)(n2- n1 +1)/2
When the line produced is called the limiting line of the series.
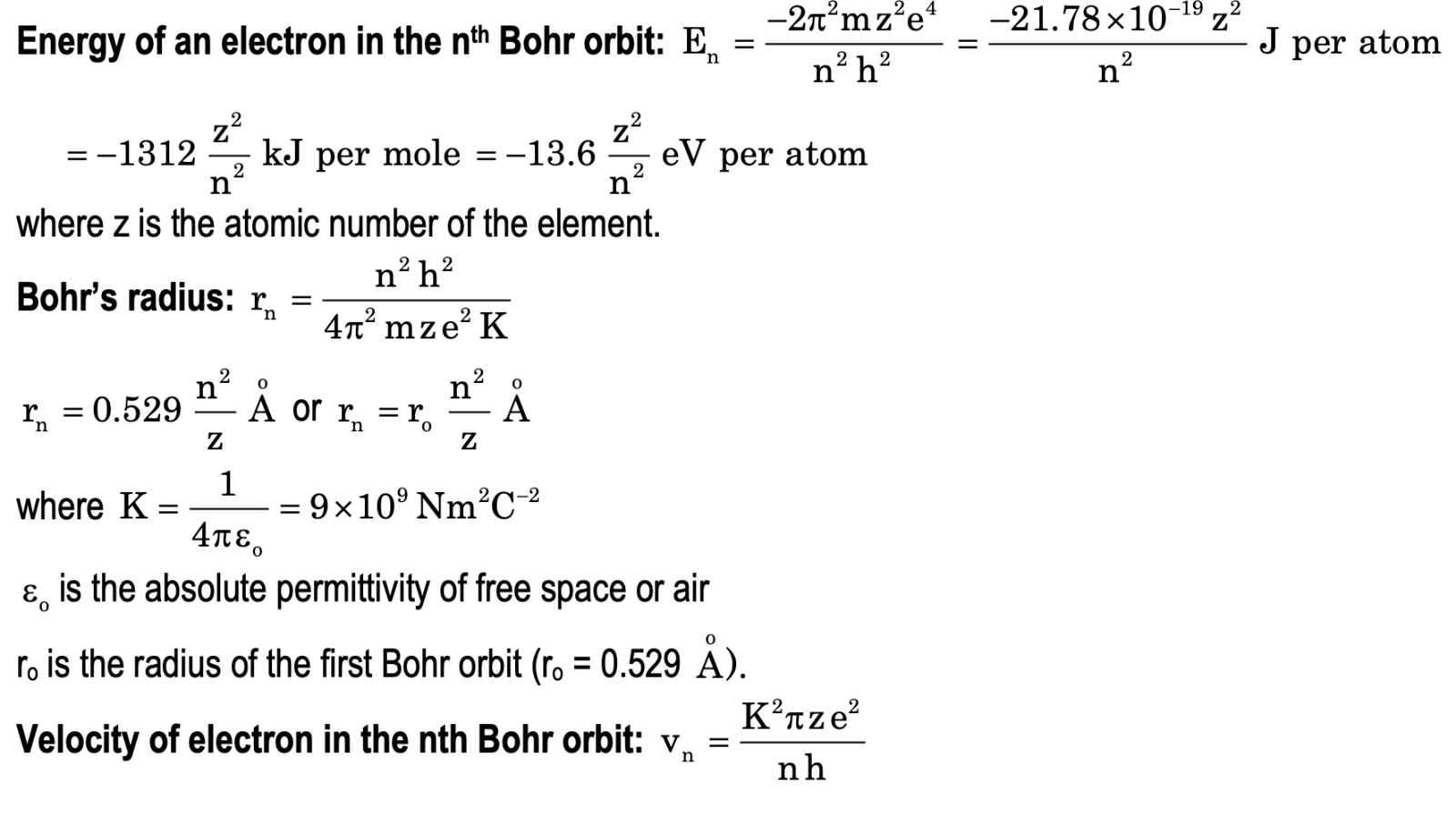
Bohr’s Model of the Atom
The main postulates of this model are:
(i) Electrons revolve only in certain orbits around the nucleus, called stationary states or energy levels, having fixed energies.
(ii) Electrons revolve in only those energy levels for which their angular momentum is an integral multiple of
 Limitations of Bohr’s theory
Limitations of Bohr’s theory
(i) It could not explain the spectrum of multi-electron atoms.
(ii) It could not explain the splitting of spectral lines into a group of fine lines under the influence of an electric field (Stark effect) and a magnetic field (Zeeman effect).
(iii) Bohr’s theory is not in agreement with Heisenberg’s uncertainty principle.
Sommerfield’s extension of Bohr’s theory
To account for the fine spectrum of H-atom - Sommerfeld proposed that electron moves in elliptical orbits and the nucleus is situated at one of the foci.
PLANCK’S QUANTUM THEORY
According to this, a hot body emits radiation energy not continuously but in small packets called quanta. Energy of each quantum is given by and for ‘n’ quantum, where is the frequency of light, ht and h is Planck’s constant, and has ing value.e
EINSTEIN’S EQUATION
It is E = mc2 , where ‘E’ is the energy of a photon, ‘m’ is the mass and ‘c’ is the velocity of photon.
PHOTOELECTRIC EFFECT
When light of a certain frequency (threshold frequency) is incident on a metal surface, electrons are ejected from the surface. If the incident radiation has a frequency, the difference in energy is converted to the kinetic energy of photoelectrons.
Where is the minimum energy required for the emission of photoelectrons is called the work function of a metal.
No electron is ejected if the energy of the incident light is less than the work function of a given metal. The number of electrons ejected depends upon the intensity and velocity, and the kinetic energy of photoelectrons depends upon the frequency of incident radiation.
DUAL NATURE OF MATTER AND RADIATION
de Broglie (1924) suggested that all material particles possess wave nature as well as particle nature and gave the equation called the de Broglie equation.
Where is the wavelength, m is mass, v is the velocity of the particle, and h is Planck’s constant.
The wave nature of electrons is confirmed by Davisson and Germer’s experiment and by Thomson’s experiment.
The particle nature is confirmed by the photoelectric effect and the scintillation method.
HEISENBERG’S UNCERTAINTY OR INDETERMINACY PRINCIPLE
It states that it is impossible to measure simultaneously the exact position and the exact momentum of a microscopic moving body. The uncertainty in position and the uncertainty in momentum are related as In other words,
where h is Planck’s constant, E is energy,y and t are time d- Broglie and the uncertainty principle both have significance only for microscopic particles and no significance in everyday life (macroscopic particles).
WAVE OR QUANTUM MECHANICAL MODEL OF ATOM
This model is proposed by Schrodinger (1920) and is based on the dual nature of the electron and Heisenberg’s uncertainty principle. He derived an equation which describes the wave motion of electrons in three-dimensional space. This is known as the Schrodinger wave equation.
wherthehe amplitude of an electronic wave is called the wave function
E: Total energy of the system
V Potential energy of electron
The square of the wave function gives the probability of finding electrons within a small three-dimensional space.
The acceptable solutions of the above equation for energy E are called eigenvalues, and the corresponding wave functions are called eigenfunctions.
Atomic Orbital
It is the three-dimensional space around the nucleus within which the probability of finding electrons is maximum.