Alkanes
Alkanes are the simplest organic compounds. They are also known as paraffins. The general formula of alkanes is CnH2n+2. Each carbon atom of alkane is sp3 hybridized and its shape is tetrahedral. The bond lengths between C-H and C-C bonds are 1.12 Å and 1.54 Å respectively. The simplest member of alkane series is methane (CH4).
Methods of Preparation
1. By catalytic hydrogenation of unsaturated hydrocarbons:
Hydrogenation takes place in the presence of finely divided nickel as catalyst at 200°C. This reaction is known as Sabatier and Sanderson's reaction.
Hydrogenation is possible at room temperature when platinum or palladium is used as a catalyst in place of Ni.
Methane cannot be obtained by this method as no unsaturated hydrocarbon contains a single carbon atom.
2. By the reduction of alkyl halides:
Alkyl halide can be reduced with Zn + CH3COOH, Zn + HCl, Zn + NaOH, Zn-Cu couple in C2H5OH, aluminium amalgam in C2H5OH or LiAlH4.
R - X + 2H ——→ R - H + HX
Alkyl halides can also be conveniently reduced by heating with HI and red phosphorous in a sealed tube.
R - I + HI ——P/150°, pressure——→ RH + I2
The function of red phosphorous is to remove iodine.
3. By the reduction of alcohols, aldehydes, ketones and fatty acids:
The above compounds and their derivatives can be reduced with hot hydroiodic acid and red phosphorous at 150°C in a sealed tube to give alkanes.
ROH + 2HI ——Red/P——→ RH + H2O + I2
RCHO + 4HI ——Red/P——→ RCH3 + 2I2 + H2O
R-COR + 4HI ——Red/P——→ R-CH2-R + H2O + 2I2
R-COOH + 6HI ——Red/P——→ RCH3 + 2H2O + 3I2
Aldehydes and ketones can also be reduced to alkanes by amalgamated zinc and conc. HCl. The reaction is known as Clemmensen reduction.
R - CHO + 2H2——Zn-Hg/HCL——→ R - CH3 + H2O
Aldehydes can be reduced to alkanes with hydrazine and KOH at 150-200°C. This reaction is known as Wolff-Kishner reduction.
CH3CHO + NH2NH2——→ CH3CH=NNH2——KOH——→ CH3CH3 + N2
4. By condensing two molecules of alkyl halides:
Two molecules of alkyl halides when treated with sodium metal in the presence of dry ether coupled to form alkane.
This reaction is known as Wurtz synthesis.
R – Br + 2Na + R – Br ——Dry ether——> R – R + 2NaBr
5. By decarboxylation of carboxylic acid:
The sodium salt of carboxylic acid is strongly heated with soda lime to give alkane by elimination of CO₂ as carbonate.
R – COONa + NaOH ——Heat/CaO——> RH + Na₂CO₃
6. Kolbe's electrolysis:
Sodium or potassium salts of fatty acids are electrolyzed to give higher alkanes at anode.
2CH₃COONa + 2H₂O ————————> CH₃ – CH₃ + 2CO₂ + 2NaOH + H₂
Methane can't be prepared by this method.
7. By action of water on aluminium carbide or beryllium carbide:
Al₄C₃ + 12H₂O ————> 4Al(OH)₃ + 3CH₄↑
Aluminium carbide
Be₂C + 4H₂O ————> 2Be(OH)₂ + CH₄↑
Beryllium carbide
Physical Properties
- State: Due to weak forces, the alkanes upto four carbon atoms are colourless, odourless gases, the next thirteen members are colourless, odourless liquids. Alkanes from C18 onwards are colourless and odourless solids.
In alkenes, except ethene, all are odourless and follow some trend as alkanes. Ethene has a pleasant odour. All are colourless. Alkynes also follow the same trend as alkanes.
- Density: The density of alkanes increases very slowly with the rise of molecular mass until it becomes constant at 0.8.
- Solubility: They are generally insoluble in polar solvents such as water but insoluble in non-polar solvents like ether, CCl4, benzene etc.
- Boiling and melting points: The boiling point of straight chain alkanes increase regularly with increasing number of carbon atoms. The melting points of alkanes do not follow a very smooth gradation with the increase of molecular size. Alkenes and alkynes also show a gradual increase in boiling and melting points with the increase of molecular mass in homologous series. They are less volatile than alkanes, i.e., their boiling point and melting point are higher than corresponding alkanes.
Chemical Properties
Alkanes are extremely stable and inert substance due to presence of non-polar C – C and C – H bonds. Alkanes are saturated compounds with strong sigma bonds which doesn’t break under ordinary conditions. Alkanes react at high temperature by free radical mechanism.
- Halogenation (free radical substitution):
Alkanes react with halogens (Cl2, Br2) in presence of light or in dark at high temperature to form corresponding substituted products.

The relative reactivity of halogens and alkanes follows this order,
F2 > Cl2 > Br2 > I2 and 3° > 2° > 1° > CH3
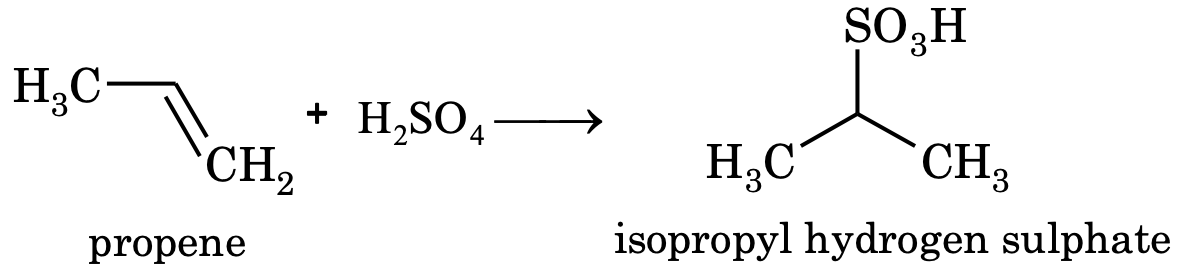
- Nitration: Nitration is possible for alkanes having three or more carbon atoms. Nitration of propane yields mixture of nitro products.
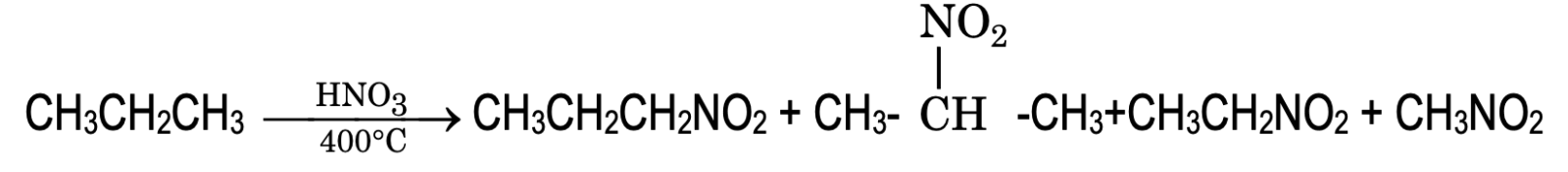
3. Sulphonation:
Higher alkanes (hexane onwards) undergo sulphonation when treated with fuming H2SO4.
n - C6H14 + HOSO3H/Hexane ——→ C6H13SO3H + H2O/Hexane sulphonic acid
4. Oxidation or combustion:
Alkanes burn in presence of O2 to form CO2 and H2O with evolution of heat.
CH4 + 2O2——→ CO2 + 2H2O
2C2H6 + 7O2——→ 4CO2 + 6H2O
ALKENES
Alkenes are characterized by the presence of a double bond between two carbon atoms. Alkenes have the general formula CnH2n.
Methods of Preparation
- By dehydration of alcohols:
Dehydration of alcohols in presence of acids forms alkene. This is elimination reaction.
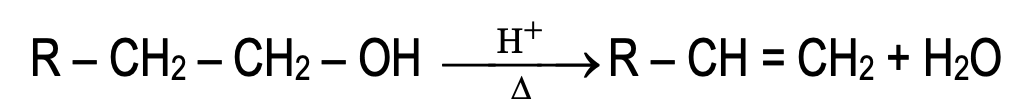
2. By the dehydrohalogenation of alkyl halides:
CH3CH2CH2Br alc. KOH → CH3CH=CH2 + HBr
If dehydrogenation of alkyl halide gives two products, the major product will be according to Saytzeff’s rule, i.e. the alkene which is most substituted is the major product.
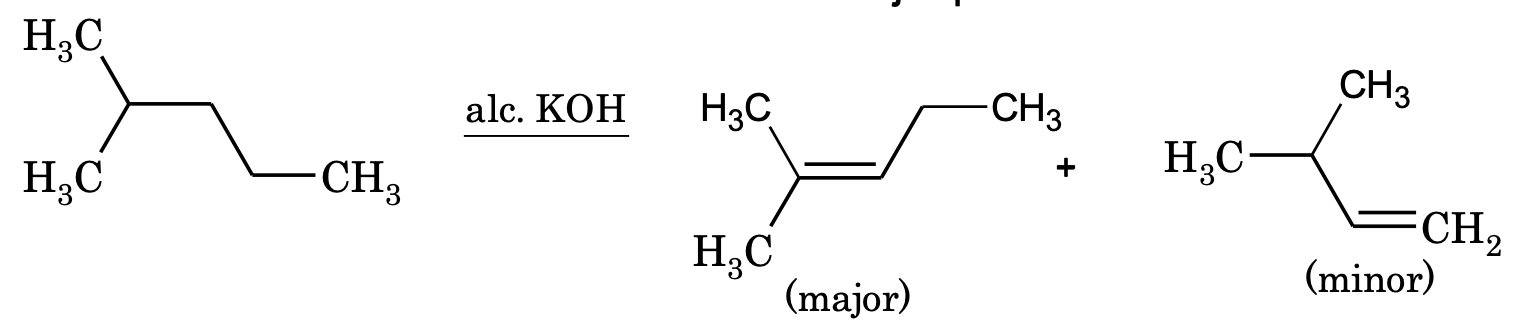
The ease of dehydrohalogenation follows the order,
Tertiary alkyl halide > secondary alkyl halide > primary alkyl halide.
Among the different halides, the order is alkyl iodide > alkyl bromide > alkyl chloride.
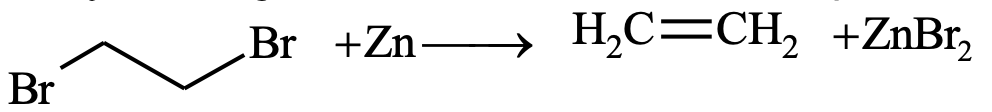
3. By the dehalogenation of vicinal dihalides:
Dehydrohalogenation of vicinal dihalides in presence of Zn dust in alcoholic solution yields pure alkene.
4. Kolbe’s electrolysis method:
The electrolysis of sodium or potassium salts of dicarboxylic acid gives alkene at anode.

However, if Na/ liq NH3 is used, trans alkene is formed, and in presence of Ni cis alkene is formed.
Chemical Properties
Alkenes are reactive due to the presence of double bonds. Due to presence of π bonds alkenes give electrophilic addition reaction. Alkenes also give free radical addition reaction.
1. Addition reactions:
(i) Addition of hydrogen (catalytic hydrogenation)
CH2 = CH2 + H2 Ni
200–300℃ → CH3–CH3
(ii) Addition of halogens (Cl2 or Br2)
CH2 = CH2 + Br2 → BrCH2–CH2Br
Ethylene dibromide (colourless)
Addition of bromine is used as a test for detecting the presence of a carbon–carbon double bond or triple bond.
(iii) Addition of hydrogen halides
CH2 = CH2 + HX → CH3CH2X
The order of reactivity among hydrogen halides is
HI > HBr > HCl > HF
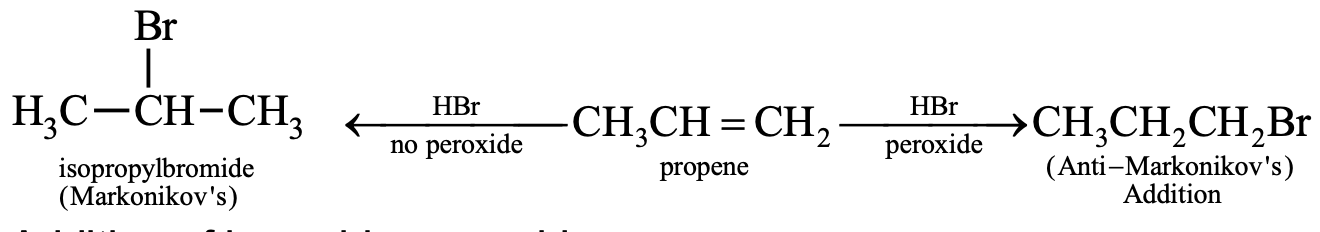
In case of unsymmetrical alkenes addition occurs according to Markonikov’s rule. This reaction takes place through an ionic mechanism. Electrophilic addition to a carbon–carbon double bond involves the formation of an intermediate, i.e. more stable carbocation.
Deviation from Markonikov’s rule:
It has been observed that addition of HBr to unsymmetrical alkenes like propene in presence of air, peroxide or light yields n-propyl bromide by anti-Markonikov’s rule. The effect is called peroxide effect or Kharasch effect.
(iv) Addition of hypochlorous acid
CH2=CH2 /Ethylene + HOCl/Hypochlorous acid → CH2OH—CH2Cl/ethylene chlorohydrin
(v) Addition of sulphuric acid
Alkyl hydrogen sulphates are water soluble, when heated at about 160ºC, they give olefins. On reaction with water they give alcohol.
CH3—CH2—OH+H2SO4 ←H2O, boil→ CH3—CH2—OSO3H 160ºC → CH2=CH2 + H2SO4
(vi) Addition of water
CH3—CH=CH2 + H2O —H+→ CH3—CH(OH)—CH3
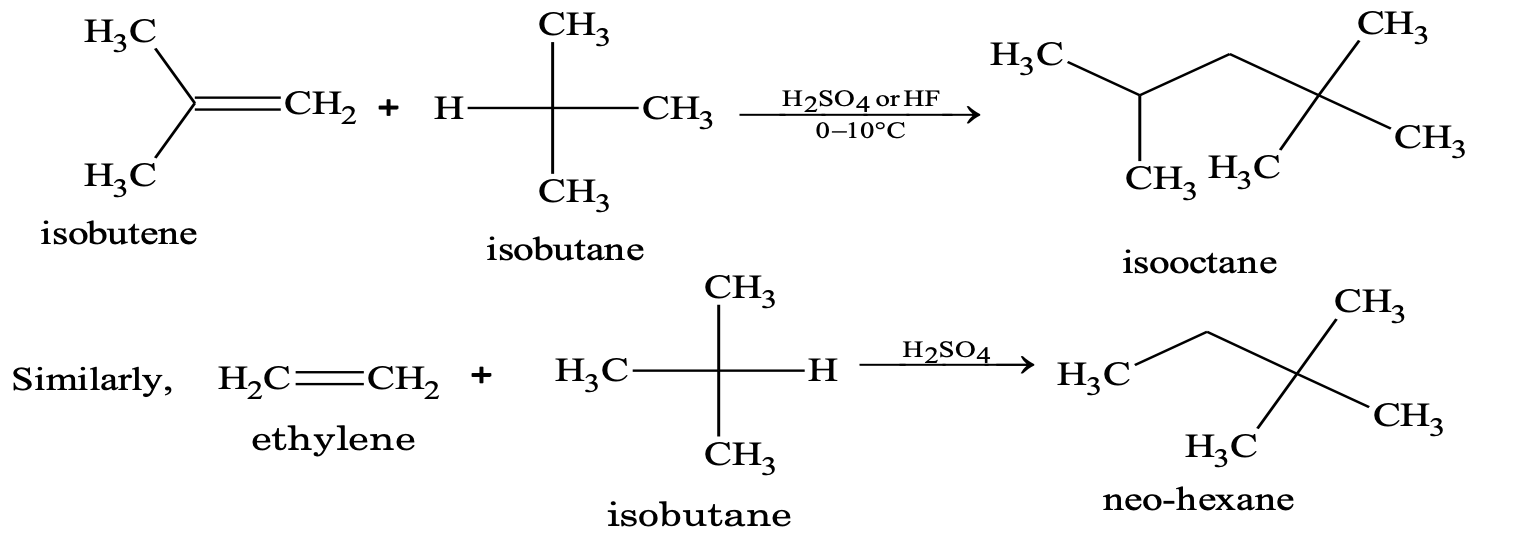
(vii) Addition of alkanes (alkylation)
(viii) Addition of diborane (hydroboration)
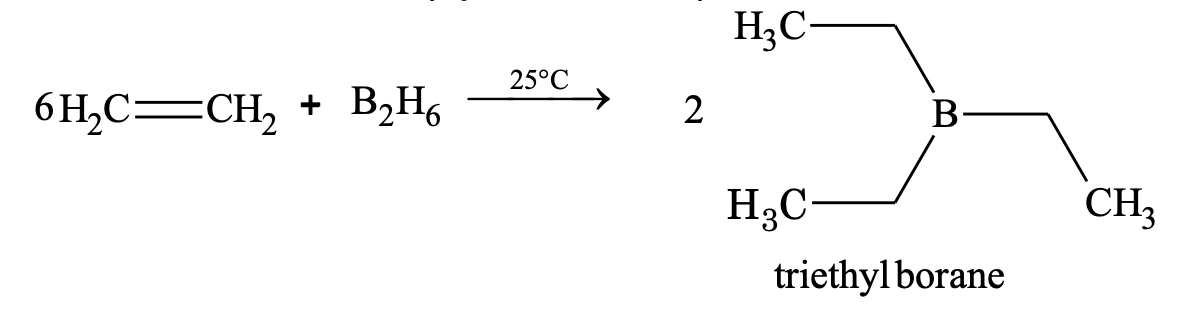
In case of unsymmetrical alkenes, addition follows the Anti Markonikov’s rule.
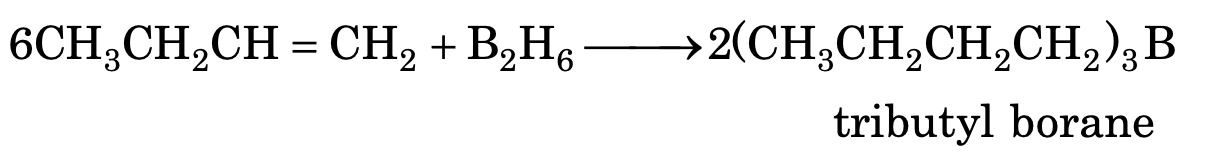
Trialkyl borane on oxidation (H2O2/OH-) gives alcohol and on reduction (LiAlH4) gives alkane.
(ix) Oxymercuration – demurcuration
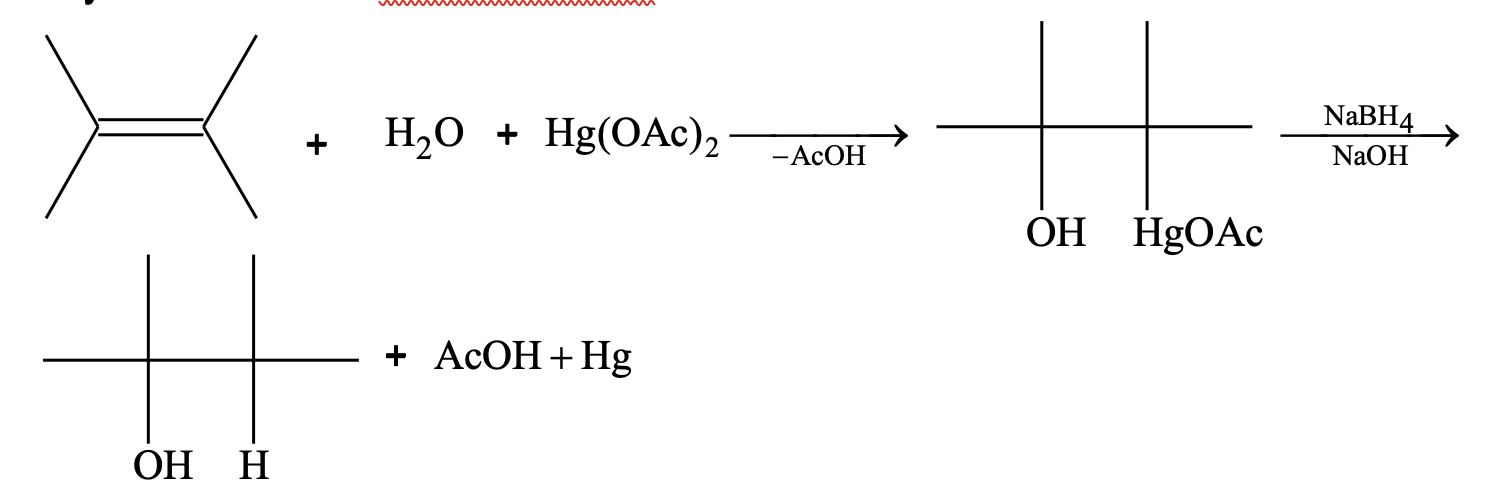
(x) Addition of oxygen
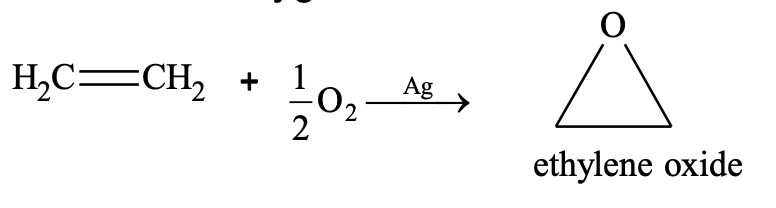
Oxidation:
(i) Oxidation by cold alkaline KMnO4 (Bayer’s reagent)
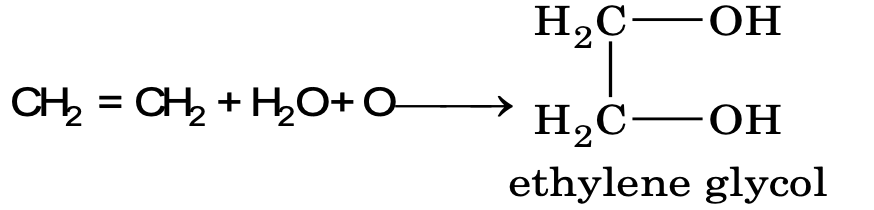
It is a test for detecting double bonds in alkene. Hydroxylation by KMnO4 is always syn addition. The cis alkene on hydroxylation gives meso compound and trans alkene gives racemic mixture. Like Bayer’s reagent OsO4 also gives glycol and the hydroxylation is syn addition.
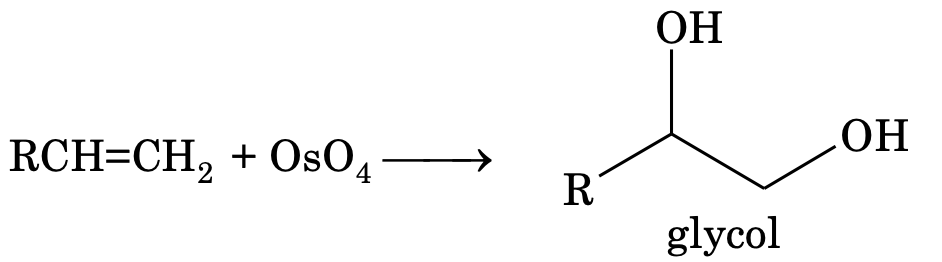
(ii) Oxidation by per acids (RCO3H)
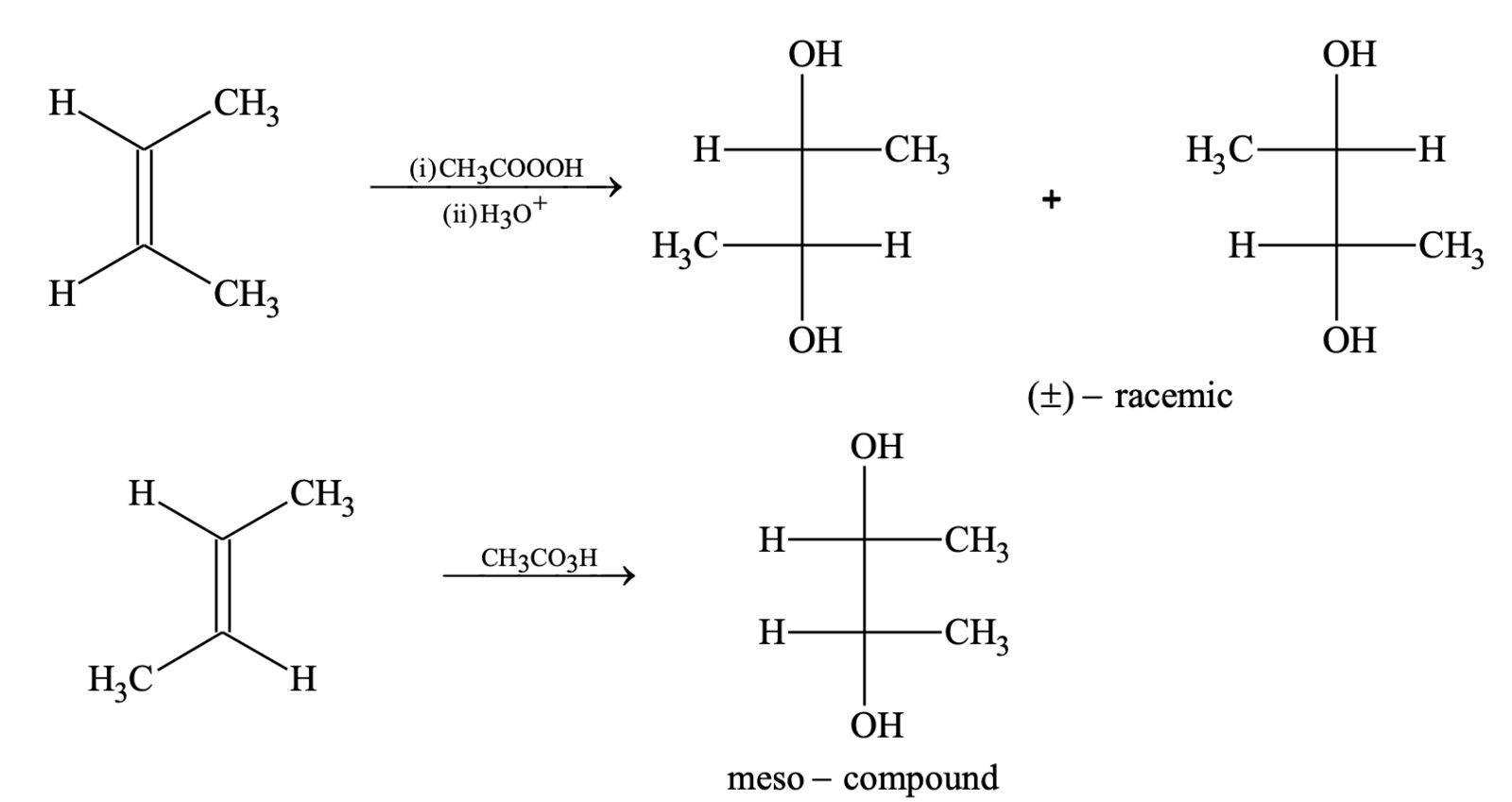
This addition occurs in trans manner. The cis alkene gives racemic mixture and trans alkenes give meso compound.
(iii) Ozonolysis
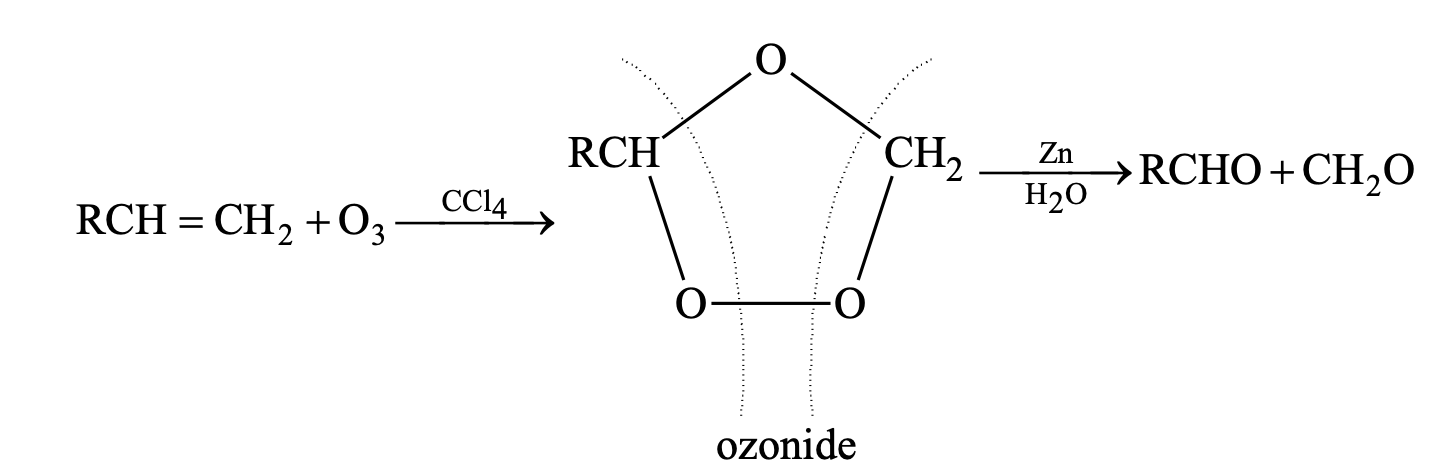
(iv) Oxidation by hot concentrated alkaline KMnO4
RCH = CH2 + KMnO4 → RCOOH + CO2 + H2O
Substitution reaction:
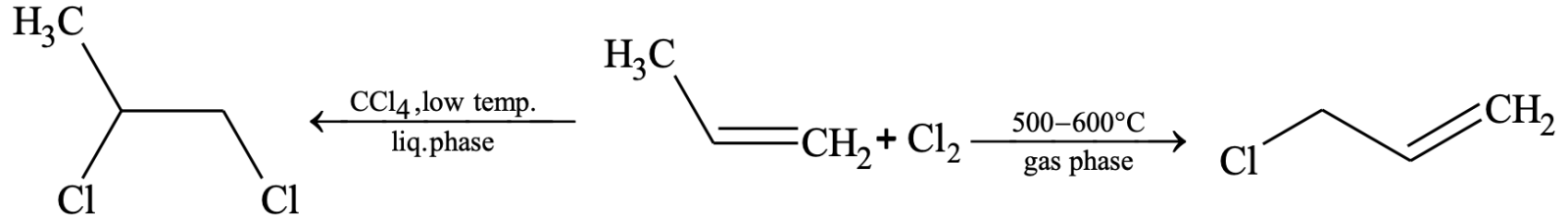
However, allylic bromination (bromination at allylic carbon atom) is very easily achieved by treating the alkene having hydrogen atom at the allylic carbon atom with N-bromosuccinimde (NBS).
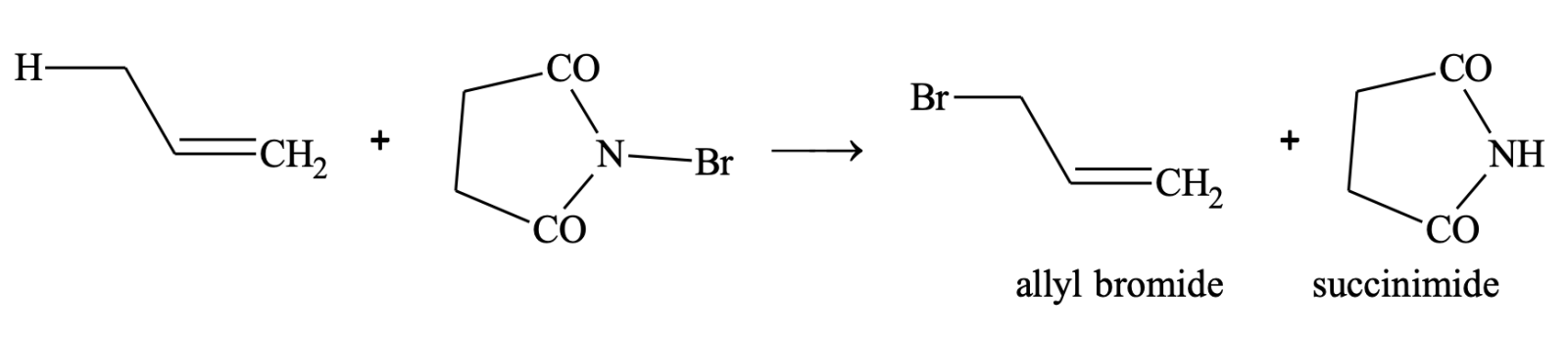
ALKYNES
Alkynes are characterized by the presence of a triple bond between two carbon atoms. The general formula of alkyne is CnH2n-2.
Methods of Preparation
1. By the dehydrohalogenation of vicinal dihalides:
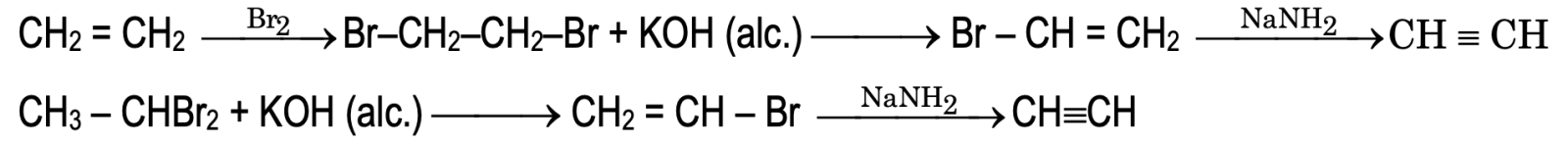
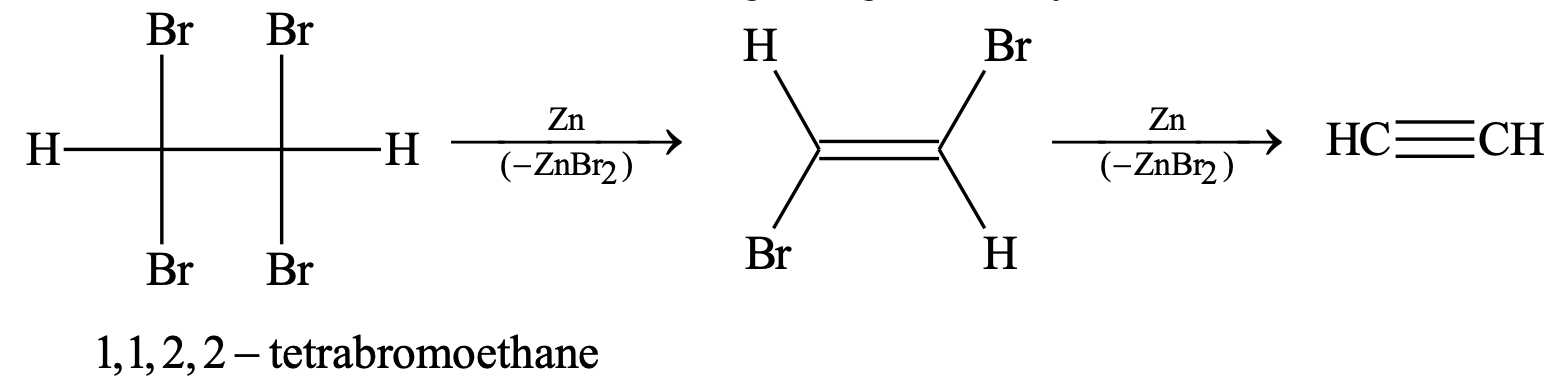
2. By dehalogenation of vicinal tetrahalides:
Reaction with active metals like Zn, Mg etc. gives acetylene.
3. By Kolbe electrolysis method:
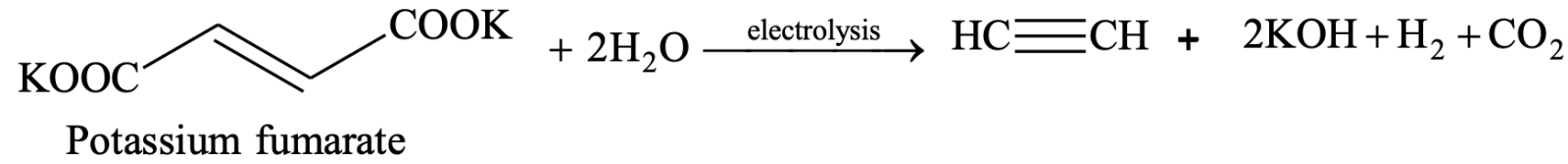
4. By heating iodoform or chloroform with silver powder or zinc:
This method can be used for the preparation of only acetylene.
CHI3 + 6Ag + CHI3 → CH≡CH + 6AgI
5. From acetylene:
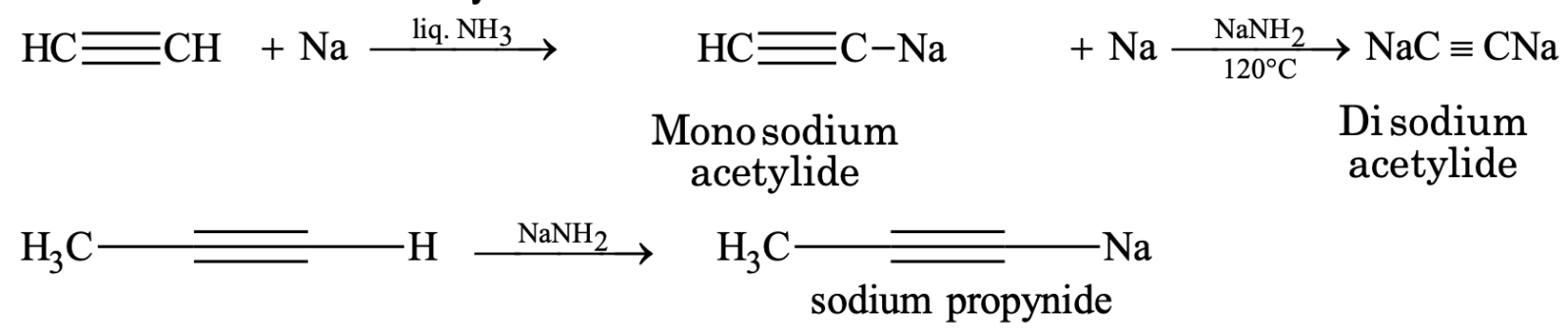
Higher alkynes can be prepared from acetylene when treated with sodium metal in liquid ammonia.
CH≡CH + Na liq. NH3 → CH≡C-Na + ½ H2
CH≡CNa + CH3Br → CH≡C–CH3 + NaBr
Sodium acetylide propyne
Similarly, CH≡CH 2Na
liQ. NH3 → NaC≡CNa 2CH3Br → CH3C≡CCH3
Chemical Properties
Alkyne gives electrophilic addition reaction due to the presence of loosely held p electrons, but electrophilic addition reactions in alkyne are slower than that of alkenes.
Terminal hydrogen present in alkynes is acidic in nature. Since, s electrons are closer to nucleus than p electrons, the electrons present in bond having more s character will be more closer to the nucleus. The amount of s character in various types of C – H bond are as follows
| Type of C–H bond | Hybrid orbital | Percentage of s character |
|---|---|---|
| ≡ C–H | sp | 50.0 |
| = C–H | sp² | 33.3 |
| - C–H | sp³ | 25.0 |
Relative acidities: HOH ≈ HOR > CH≡CR > :NH₃ > CH₂=CH₂ > CH₃–CH₃
Relative basicities: OH⁻ ≈ OR⁻ < C≡C⁻R < NH₂⁻ < CH⁻=CH₂ < CH₂⁻–CH₃
1. Addition of hydrogen:
CH≡CH + H2 Ni/acetylene → CH2=CH2 / ethylene —H2→ CH3–CH3 / ethane
In case of alkynes where triple bond is not present at the end of the chain, on reduction gives cis or trans alkene, which depends upon the choice of reducing agent. With sodium in liquid ammonia the alkene is trans form and on catalytic reduction the alkene is cis form.
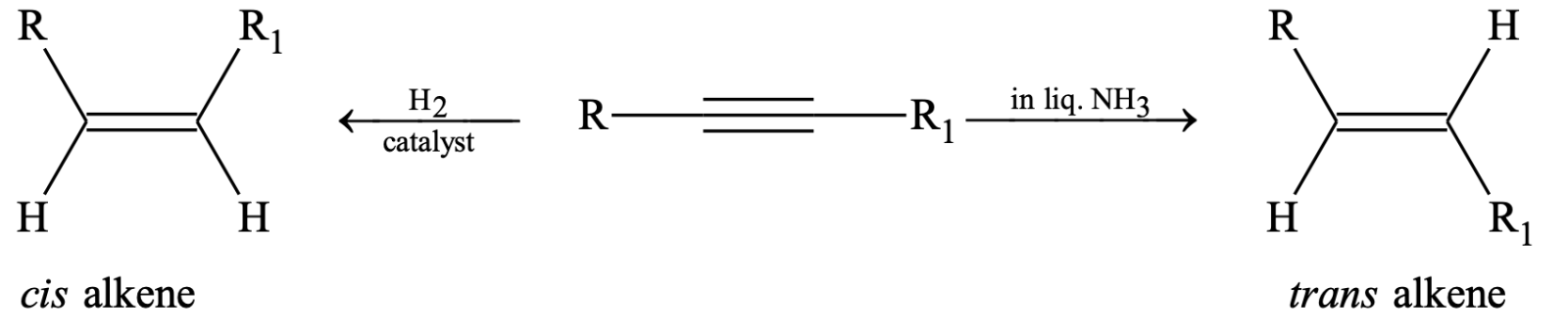
Electrophilic addition:
(i) Addition of halogens
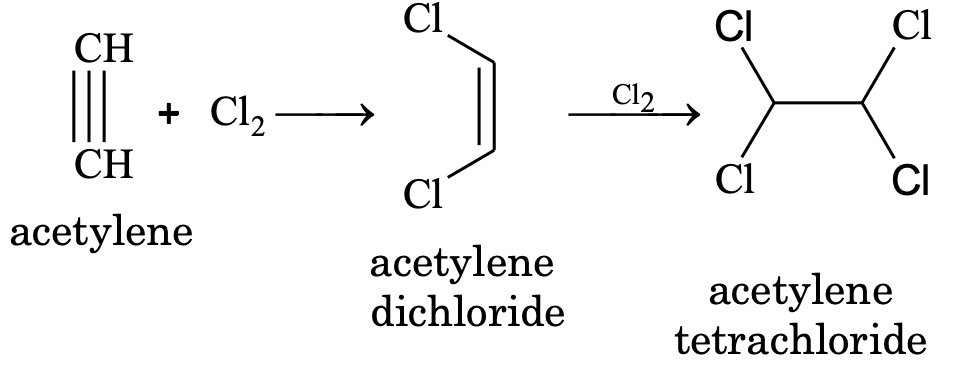
The order of reactivity of halogens is Cl2 > Br2 > I2
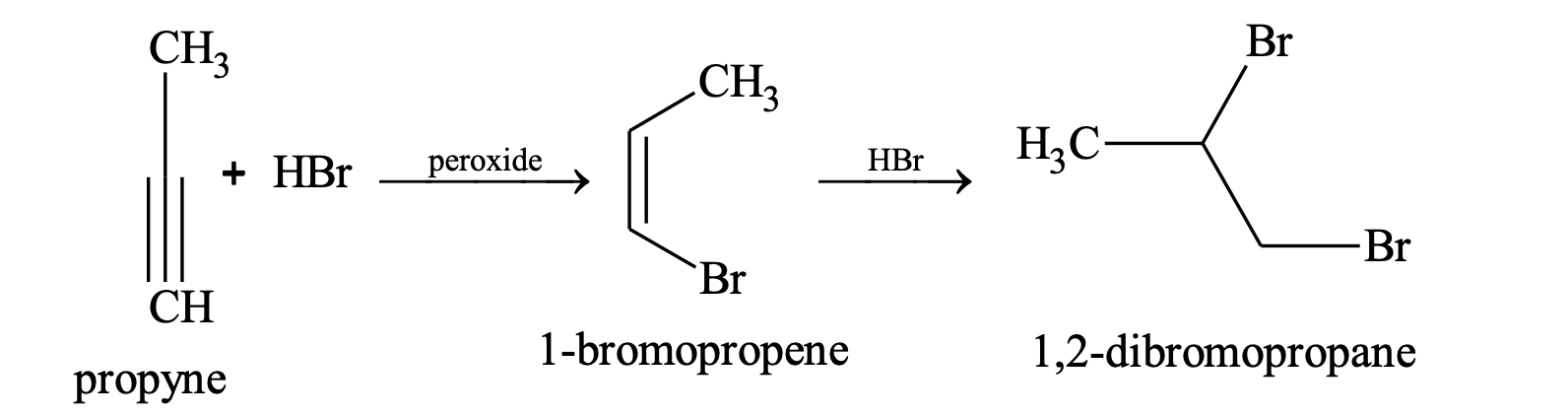
(ii) Addition of halogen acid
The order of reactivity of halogen acids is HI > HBr > HCl.
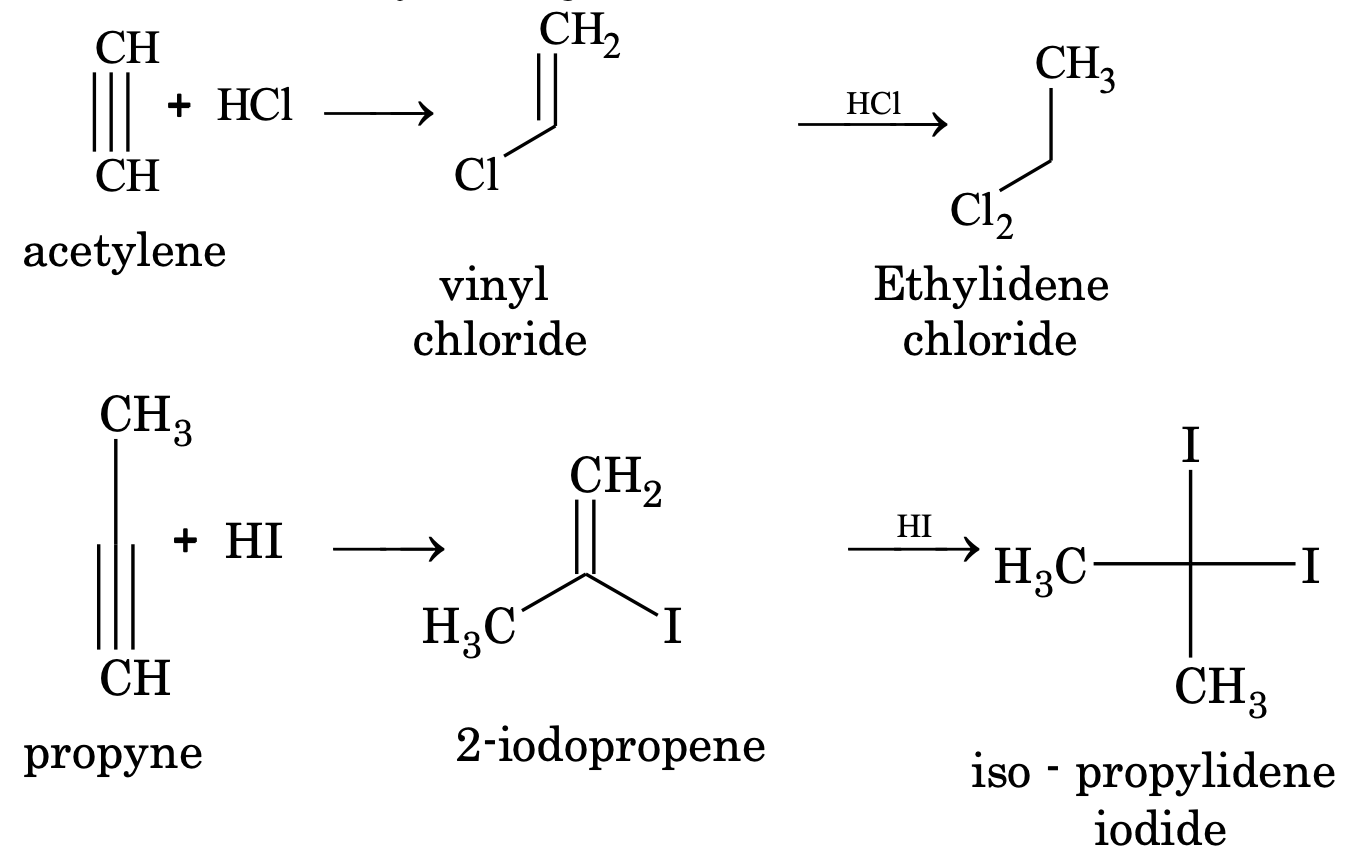
In presence of peroxide, anti Markonikov’s product is obtained.
(iii) Addition of hypohalous acids
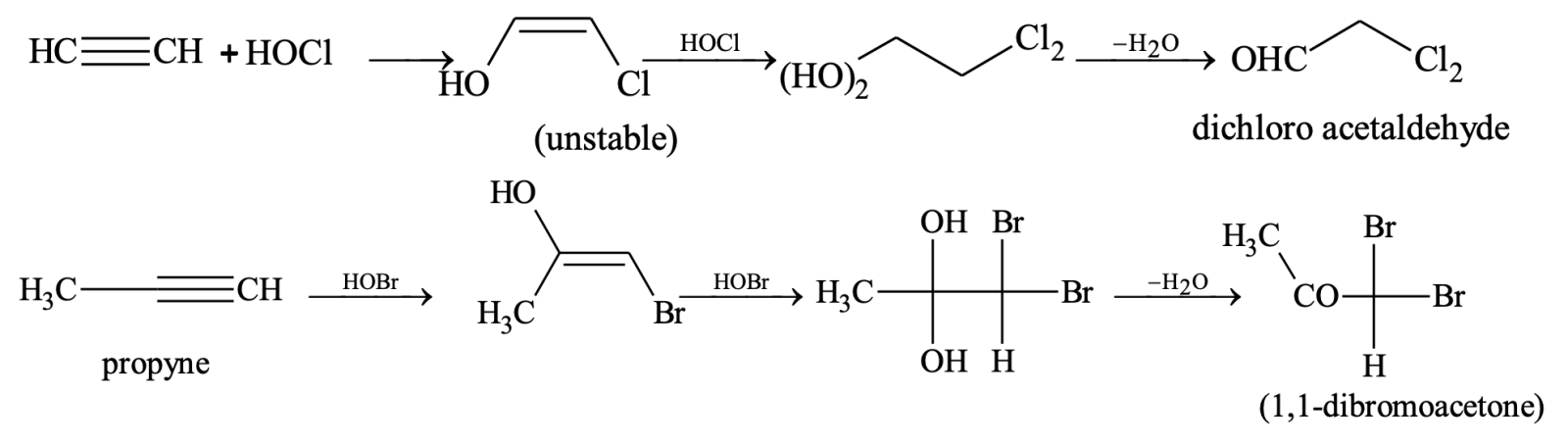
Nucleophilic addition reaction:
In these reactions, the addition is initiated by a nucleophile and are generally catalysed by salt of heavy metals (e.g. Hg2+, Pb2+, Ba2+) which are found to form p compound with multiple bonds.
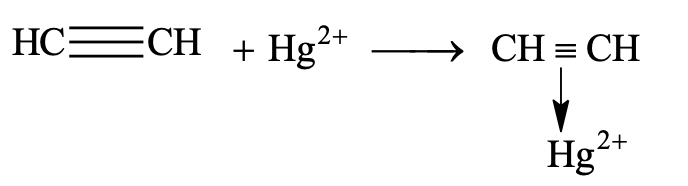
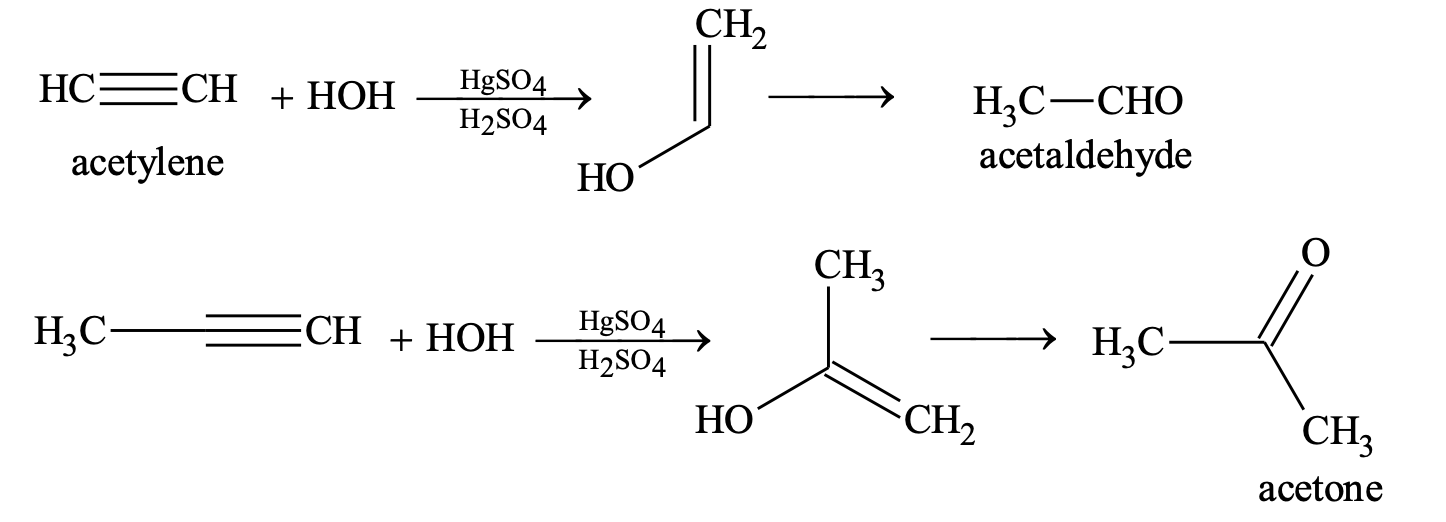
(i) Addition of water
(iv) Addition of alcohol
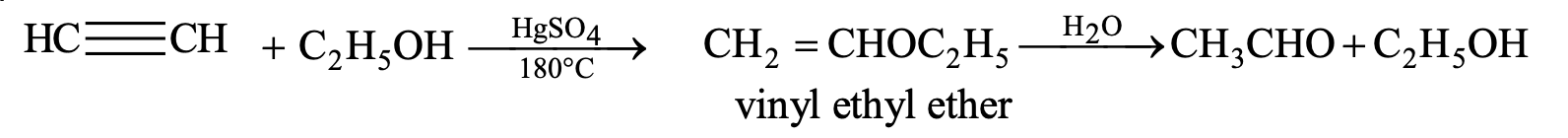
(v) Addition of ozone and ozonolysis
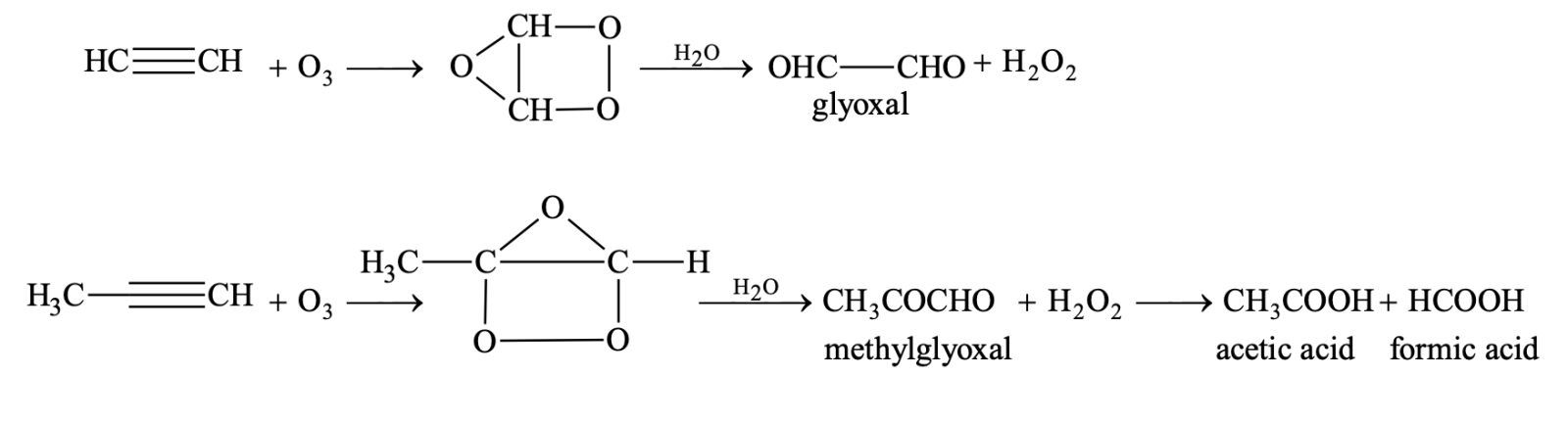
Oxidation:
(i) Oxidation with alkaline KMnO4
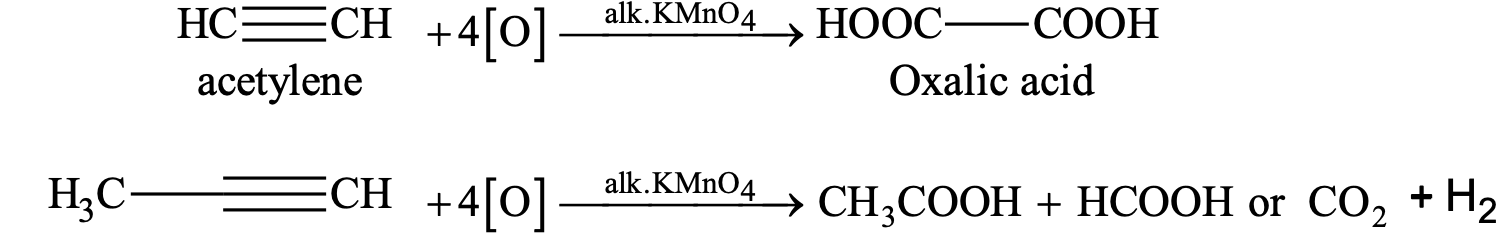
(ii) Oxidation with acidic K2Cr2O7 or KMnO4
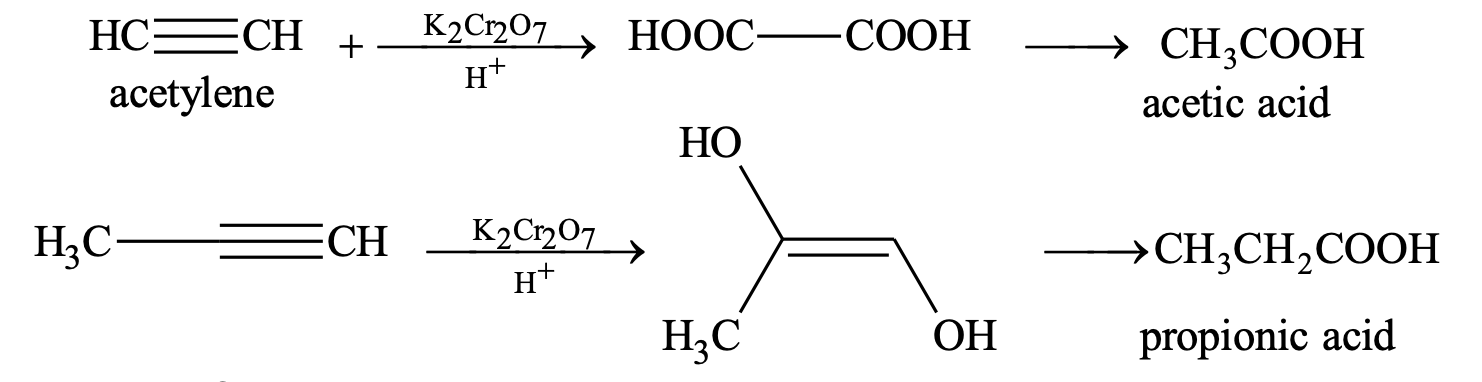
5. Formation of metallic derivatives:
The group –CºC–H in alkynes is slightly acidic in nature and hence its hydrogen atom can be easily replaced by certain metals to give metallic derivatives called acetylides or alkynides.
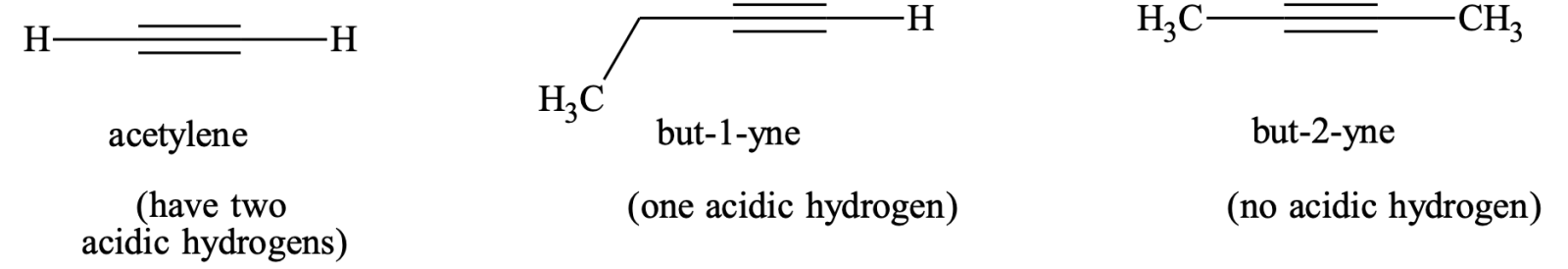
(i) Formation of sodium acetylides
(ii) Formation of copper and silver acetylides
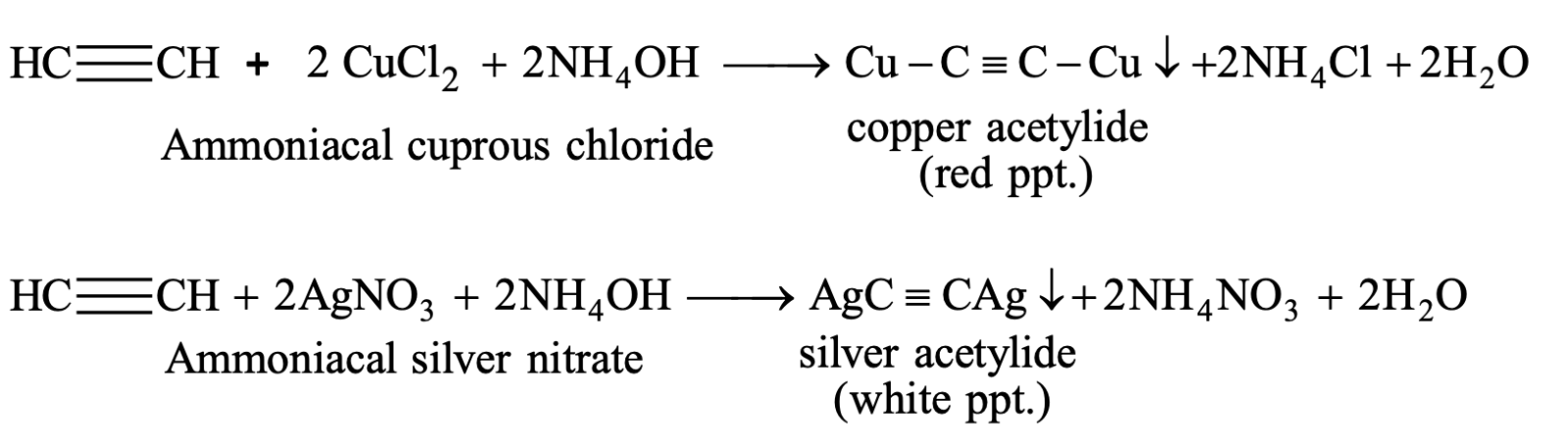
These reactions are used for detecting the presence of acetylinic hydrogen atom.