Struggling to remember the names of organic compounds or keep track of IUPAC rules? You’re not alone!
At Home- Tuition, we turn chemistry confusion into confidence. Get expert-led, step-by-step lessons, quick doubt clearance, and powerful tips to ace nomenclature for board exams, JEE, NEET, and more.
Build Your Chemistry Foundation—Understand, Don’t Just Memorize!
Learning organic chemistry nomenclature isn’t just about memorizing names; it’s about understanding the logic behind every compound. Mastering IUPAC nomenclature opens doors to better exam scores, a clearer understanding of reactions, and lifelong confidence in chemistry.
Whether you’re facing your CBSE boards, JEE, NEET, or just want to strengthen your basics, our resources ensure you truly “get” nomenclature, not just cram it.
What You’ll Learn: Step-by-Step Lessons, From Basics to Advanced
We make organic compound naming simple, even if you’re starting from scratch. Our curriculum covers:
- IUPAC rules and naming conventions—from selecting the parent chain to assigning priorities and suffixes.
- Functional group identification and priority order—never get confused by what comes first in a name.
- The lowest sum rule and numbering system—clear steps for accurate, exam-accepted answers.
- Naming tricky compounds: Alkyl halides, aromatic compounds, iso/neo naming, and more.
- Hands-on practice: Real-life examples, solved exercises, and exam-style questions.
- Special strategies for board and entrance exams: Tailored tips for CBSE, ICSE, JEE, and NEET aspirants.
Every session includes cheat sheets, visual guides, and memory hacks to help you master nomenclature faster.
Why Choose Home Tuition?
- Trusted by Toppers: 5+ years of academic success and thousands of happy students.
- Expert Faculty: Teachers who specialize in chemistry and understand student challenges.
- Proven Results: Consistent score improvements and top ranks in CBSE, JEE, NEET, and Olympiads.
- Flexible Learning: Join live or recorded sessions at your convenience.
- Student-First: Personalized attention and practical solutions for every learner.
INTRODUCTION
Nomenclature of organic compounds
Organic chemistry is the study of hydrocarbons and their derivatives. The term nomenclature means the system of naming of organic compounds. In case of aliphatic compounds, two systems of nomenclature are generally used
(A) Trivial or common system and
(B) IUPAC system
(A) Trivial or Common System: In the beginning, when the number of carbon compounds were limited, they were named on the basis of their sources, properties and structures eg.
(i) From sources: Urea (from urine) Methanol (from wood)
(Greek, methu = spirit, hule = wood)
(ii) From properties: Glucose (from Greek, glykys = sweet), olefin (from French, olefiant = oil forming)
(iii) From structures: n-petane (from Greek = penta = fuie), methyl chloride (from methyl group + chloride)
When all the carbon atoms in a hydrocarbon chain are joined together in a straight chain, it as called n-alkane, if (CH3)2CH¾group is attached with a straight chain, it is called iso alkane and when a (CH3)3 C- group is joined with a straight chain, it is called as neo-alkane.
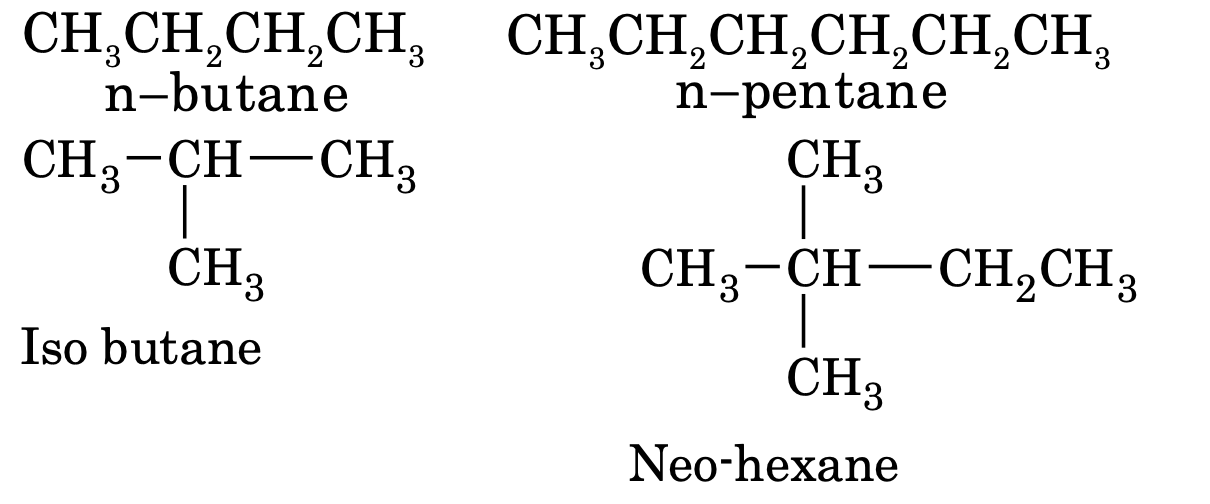
(B) IUPAC System
Due to property of catenation and isomerism, carbon forms a large number of organic compounds. Hence, it became quite difficult to remember them by their common or trivial names. In order to systematize, the nomenclature of organic compounds, IUPAC (International Union of Pure and Applied chemistry) system of nomenclature was first introduced in 1947. These rules underwent modifications from time to time and the most exhaustic rules for nomenclature of organic compounds were first published in 1979 and later revised and updated in 1993 (which are to be followed in this chapter) and is widely used throughout the world.
General rules for IUPAC nomenclature
The most important feature of this system is that any given molecular structure has only one IUPAC name and any given IUPAC name denotes only one molecular structure.
The IUPAC name of any organic compound essentially consists of three parts i.e.
(i) Word root
(ii) Suffix and
(iii) Prefix
(i) Word root:
It is the basic unit of the name which denotes the number of carbon atoms in the longest possible continuous chain.
|
Chain length |
Word root |
Chain length |
Word root |
|
C1 |
Meth |
C6 |
Hex |
|
C2 |
Eth |
C7 |
Hept |
|
C3 |
Prop |
C8 |
Oct |
|
C4 |
But |
C9 |
Non |
|
C5 |
Pent |
C10 |
Dec |
|
C11 |
Undec |
||
|
C12 |
Dodec |
(ii) Suffix. There are of two types.
(a) Primary suffix:
A primary suffix is added to word root to indicate whether the carbon chain is saturated or unsaturated. If the parent chain contains two, three or more double or triple bonds then prefixes such as di (for two), tri (for three), tetra (for four) etc are added before primary suffix.
|
Type of carbon chain |
Primary suffix |
General name |
|
|
(a) |
Saturated |
- ane |
Alkane |
|
(b) |
Unsaturated with one double bond |
- ene |
Alkene |
|
(c) |
Unsaturated with one triple bond |
- yne |
Alkyne |
|
(d) |
Unsaturated with two double bonds |
- diene |
Alkadiene |
|
(e) |
Unsaturated with three double bonds |
- triene |
Alkatriene |
|
(f) |
Unsaturated with two triple bonds |
- diyne |
Alkadiyne |
(b) Secondary suffix:
Secondary suffix are added to primary suffix to indicate the different functional groups present in the organic compound. If two or more functional group is present then the main is suffixed and others are prefixed. Secondary suffixes of some important functional groups are given below.
|
Organic compounds |
Functional group |
Prefix |
Suffix |
Example |
|
Alcohols |
- OH |
Hydroxy |
- ol |
CH3OH (Methanol |
|
Aldehyde |
- CHO |
Aldo/formyl |
- al |
CH3CHO (Ethanal) |
|
Ketones |
>C = O |
Oxo / keto |
- one |
CH3COCH3 (Propanone) |
|
Carboxylic acid |
- COOH |
Carboxy |
- oic acid |
CH3COOH (Ethanoic acid) |
|
Acid chlorides |
- COCl |
Chlorocarbonyl |
- oyl chloride |
CH3COCl (Ethanoyl chloride) |
|
Esters |
- COOR |
Carbalkoxy |
alkyl – oate |
CH3COOCH3 (Methyl ethanoate) |
|
Acid amide |
- CONH2 |
Carboxamide Carbamoyl |
amide |
CH3CONH2 (Ethanamide) |
|
Thioalcohols |
- SH |
Mercapto |
- thiol |
CH3SH (Methane thiol) |
|
Ether |
- OR |
Alkoxy |
- |
CH3OCH3 (Methoxy methane) |
|
Amines |
- NH2 |
Amino |
amine |
CH3CH2NH2 (Amino ethane) |
|
Nitriles |
- CN |
Cyano |
- nitriles |
CH3CN (Ethane nitrile) |
|
Iso nitrile |
-N≡C |
Isocyano |
isonitrile |
CH3 NC(Methyl isocyanide) |
|
Sulphonic acids |
- SO3H |
Sulpho |
Sulphonic acid |
CH3SO3H (Methyl Sulphonic acid) |
Note:
While adding the secondary suffix to the primary suffix, the terminal ‘e’ of the primary (i.e, ane, ene yne) is dropped if the secondary suffix begins with a vowel but it is retained if the secondary suffix begins with a consonant.
(c) Prefix:
These are of two types.
(I) primary prefix.
A primary prefix is used to simply distinguish cyclic from acyclic compounds.
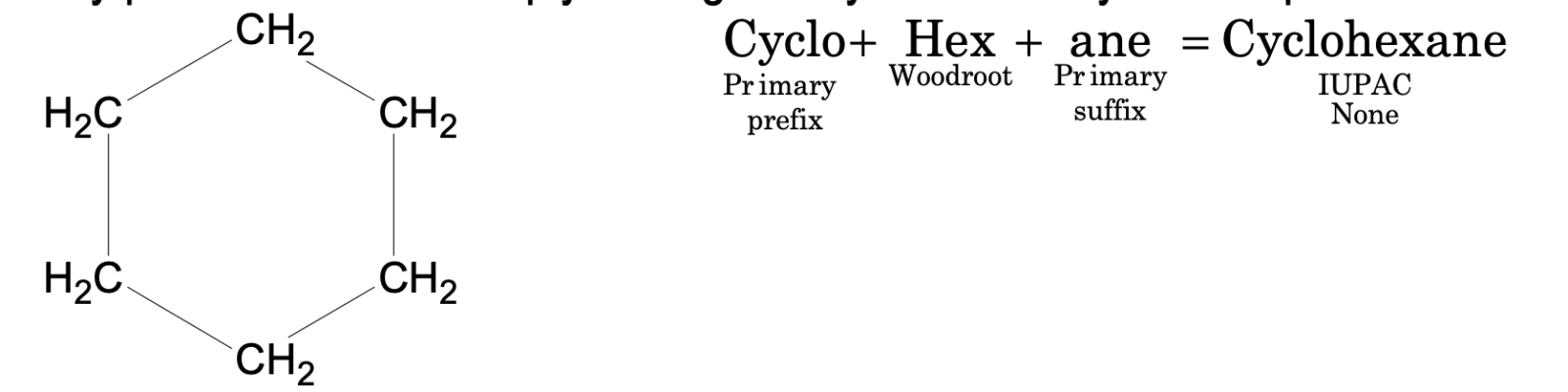
If the prefix cyclo is not used, it simply indicates that the compound is acyclic or open chain.
(II) Secondary prefix:
In IUPAC system, Certain groups are not considered as functional groups but instead are treated as substituents. These are called secondary prefixes are added just before word root or before primary prefix in cyclic compounds, in alphabetic order.
The secondary prefixes of some groups which are always treated as substituents irrespective of the fact that the compound is mono functional or poly functional are listed below.
|
Substituent group |
Secondary prefix |
Substituent group |
Secondary prefix |
|
- F |
Fluoro |
||
|
- Cl |
Chloro |
- R |
Alkyl |
|
- Br |
Bromo |
||
|
- I |
Iodo |
- CH2 CH2 CH3 |
n–propyl |
|
- NO2 |
Nitro |
- CH (CH3)2 |
Iso–propyl |
|
- NO |
Nitroso |
- C(CH3)3 |
t–Butyl |
| -N+ ≡ N |
Diazo |
||
|
- OR |
Alkoxy |
||
| -c - C - O |
Epoxy |
In case of polyfunctional compounds, the functional groups are written in prefix which all not considered main functional group in alphabetical order.
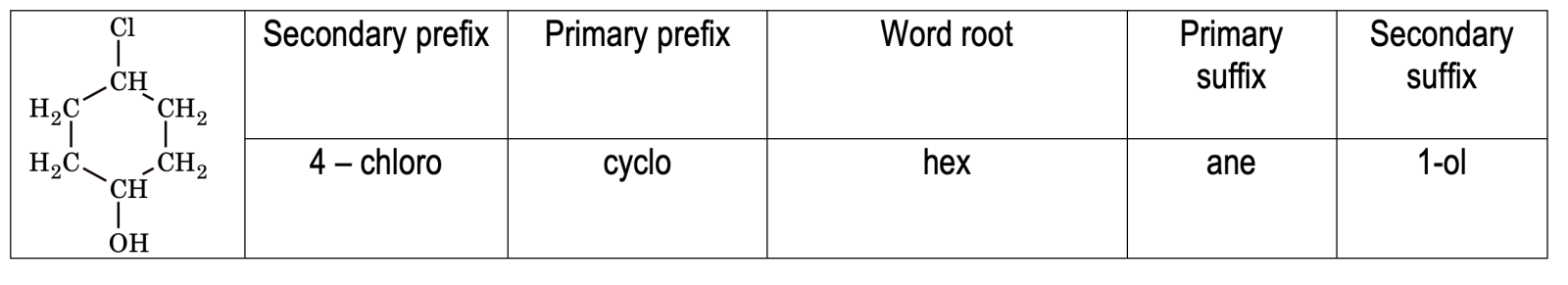
Thus, a complete IUPAC name of an organic compound consists of
Secondary Prefix + Primary Prefix + Word root +Primary suffix + Secondary suffix
e.g.
Rules for naming aliphatic organic compounds by IUPAC system – Saturated Hydrocarbons (Alkanes)
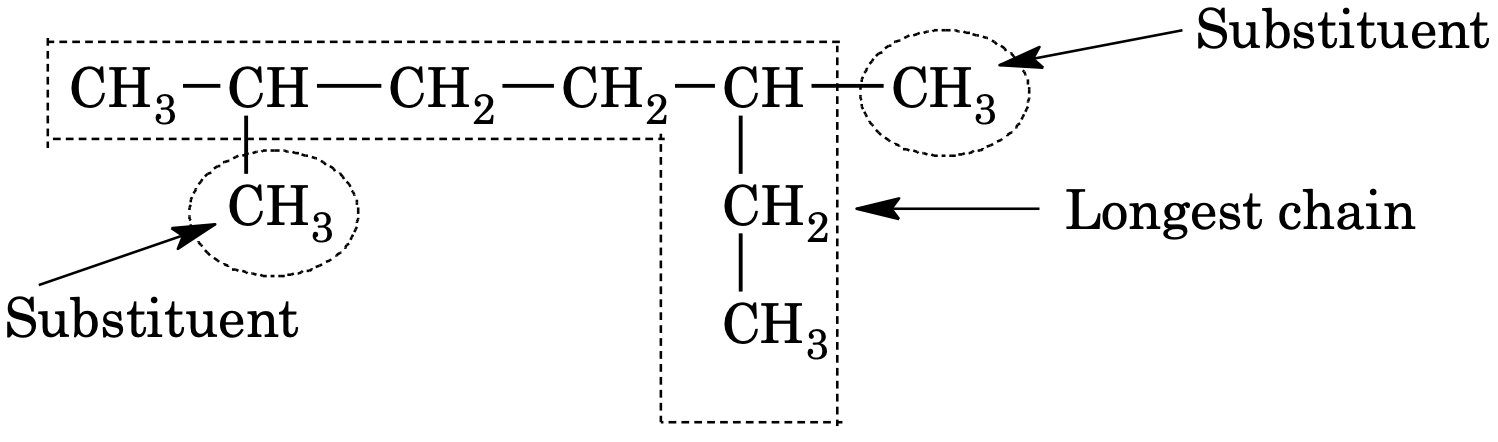
(1) Longest chain:
The longest continuous carbon chain is selected which may or may not be straight. If the main chain contains some branch or side chains, they are regarded as substituents and named in prefixes in alphabetical order, e.g.
(2) If two or more chains containing equal number of carbon are possible, the selected chain must be that which contains maximum number of side chain or substituents. e.g.
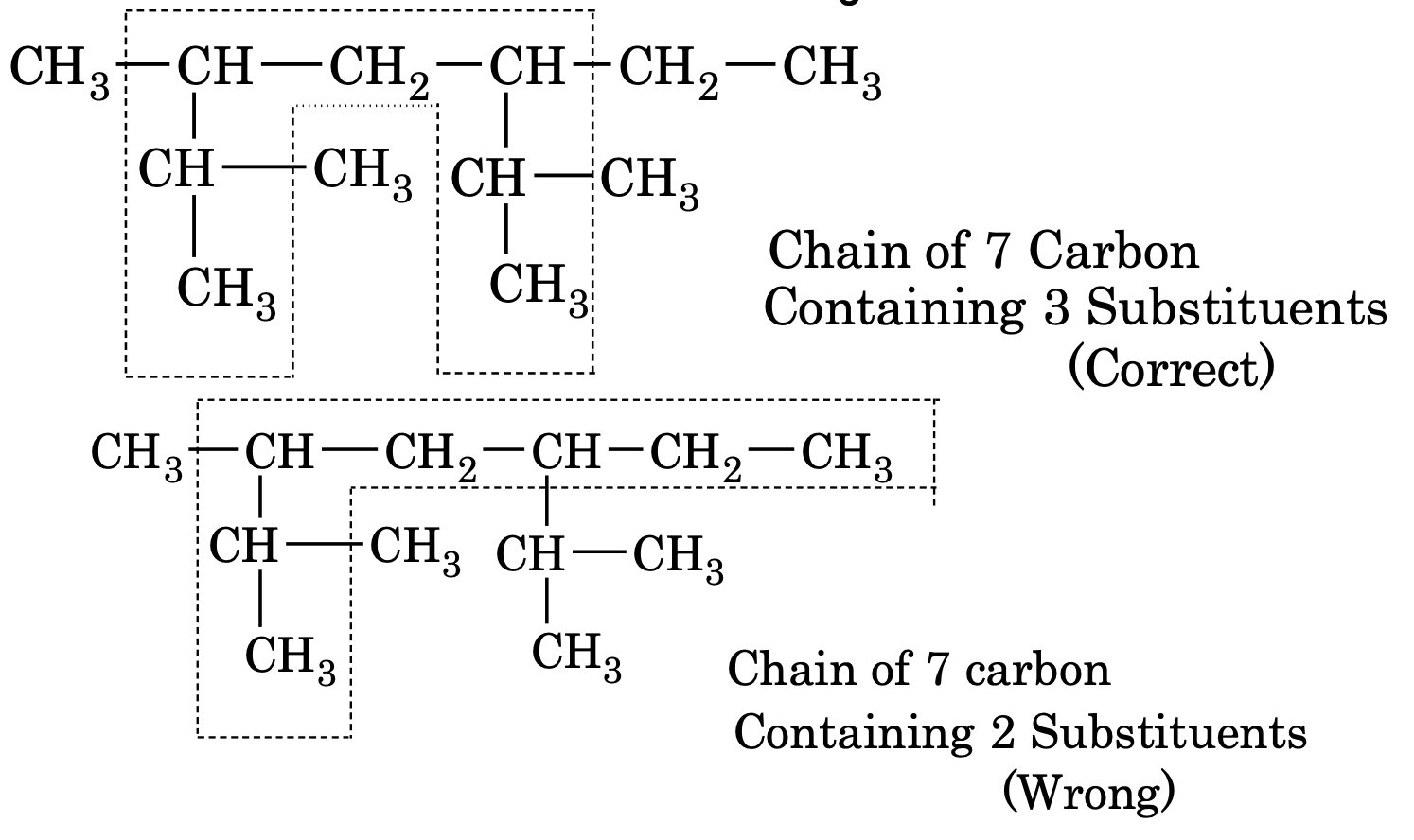
(3) Lowest Number or Lowest Sum rule.
The parent carbon chain is numbered. The number indicates the position of the Substituent on the parent chain and is called positional number or the locant.
The numbering is done in such a way to give minimum number to the Substituent (When two or more Substituent are present on parent chain)
The numbering can be done following either by Lowest sun rule or lowest set of Locants rule.
(a) Lowest Sun rule:
When two or more substituents are present, the numbering of the carbon atoms of the parent chain is done in such a way that the sum of locants is the lowest. This is called lowest sum rule.
(b) Lowest set of locants rule:
When two or more substituents are present, the carbon atoms of parent chain are numbered in such a way that the set of locants is the lowest. This is called lowest set of locants rule. The set from either direction are then compared term by term till the first point of difference is reached. That set of locants is preferred which has a lowest number at the first point of difference.
Example:
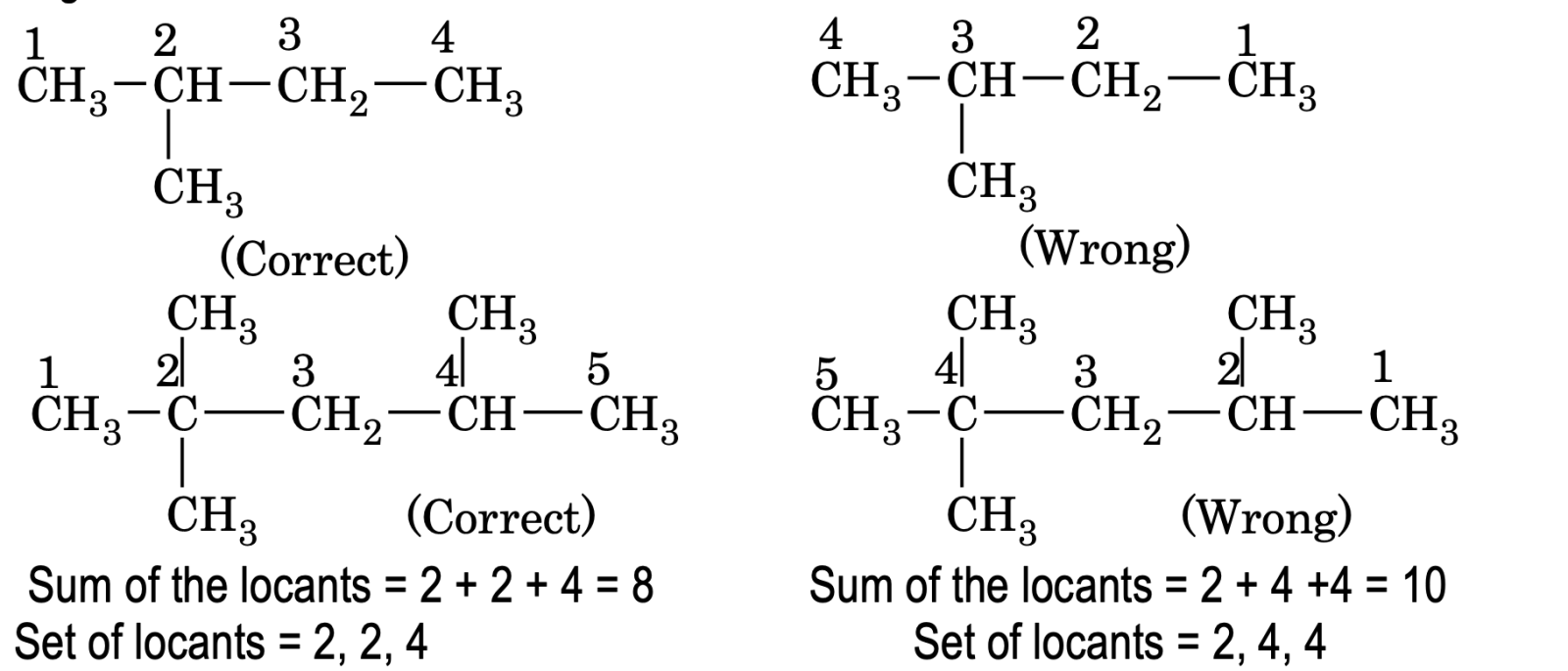
When the length of the carbon chain is small, both lowest sum rule and lowest number rule gives the same result but when the carbon chain is long differences may arise.
In such cases, according to IUPAC Nomenclature, the lowest set of locant rule should be preferred over the lowest sum rule ie lowest set of locants should be applied in general. e.g.
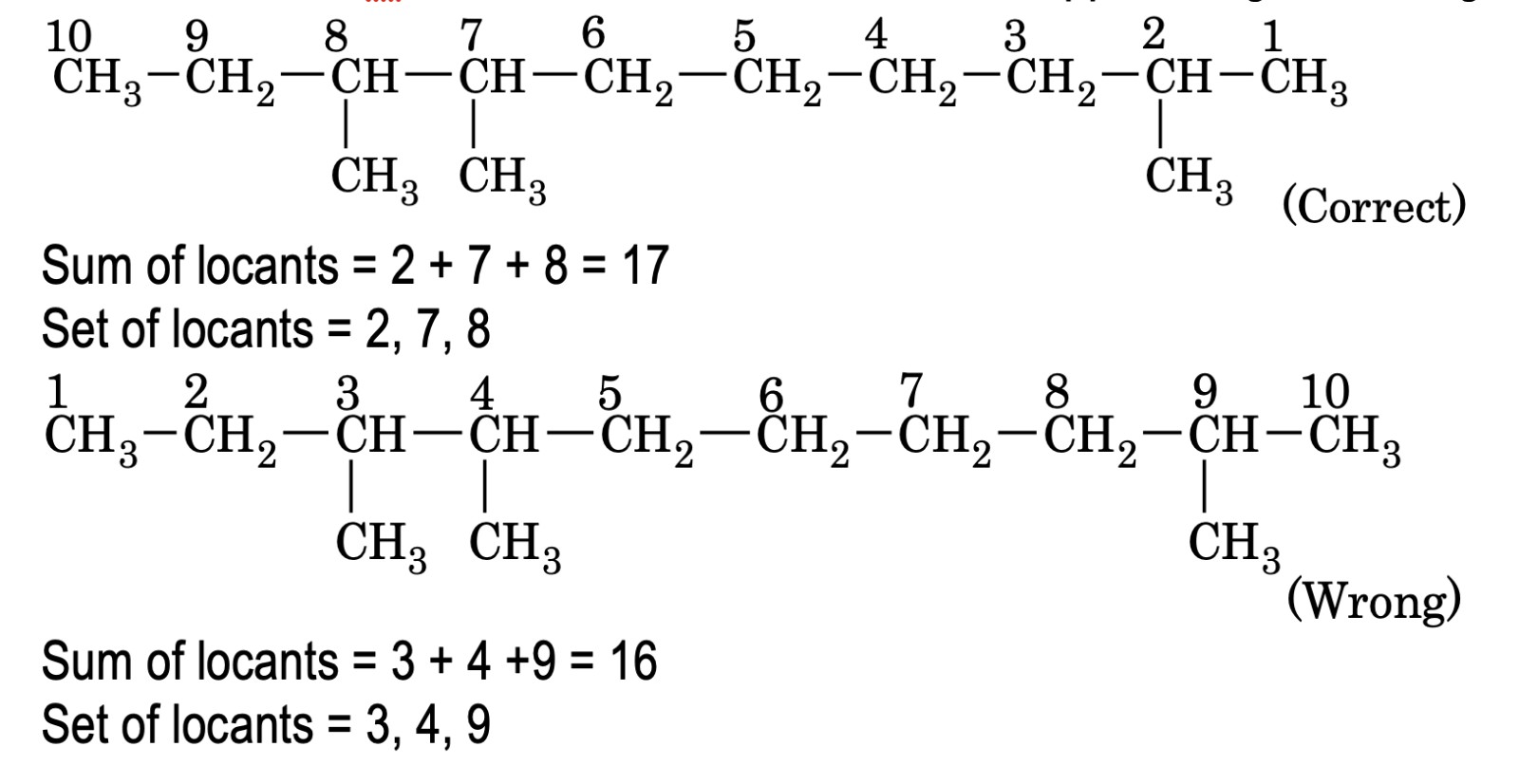
(4) Naming the hydrocarbons.
The parent alkane name is written with the substituents in prefix along with its locant, separated by a hyphen (–). The whole alkane is always written as one word.
e.g.
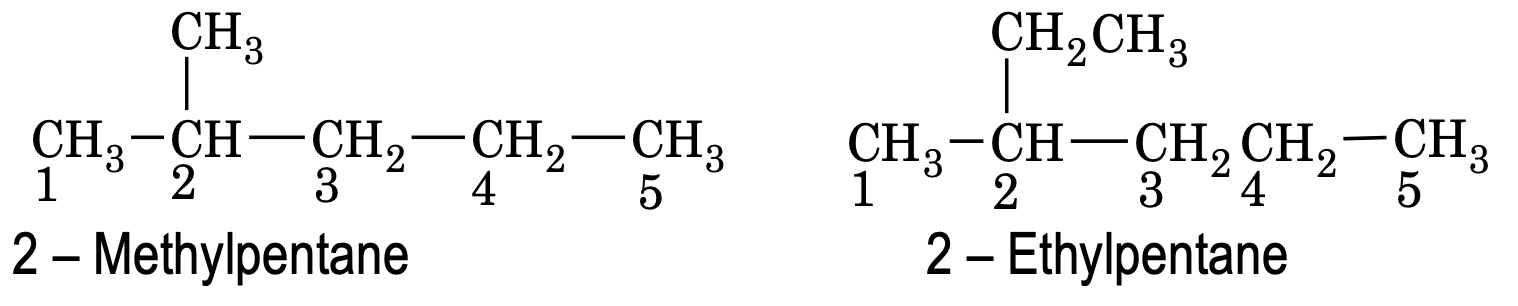
(5) Naming of alkane having same Substituent at different positions.
If the compound contains more than ore similar alkyl groups, their positions are indicate separately and an appropriate numerical prefix, di (for two), tri (for three) etc, is attached to the name of the substituent. The portions of substituents are separated by commas.
If more than one similar alkyl groups or substituents are present at same position, their number is also repeated.
e.g.
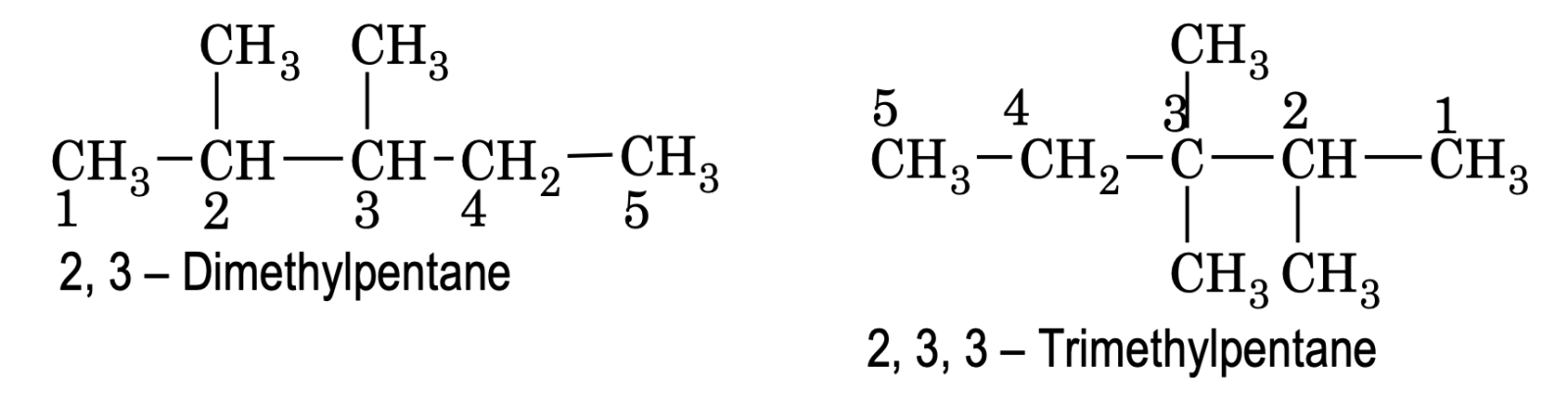
(6) Naming of compound containing different substituents.
(a) When two or more different substituents are present, the naming is done in alphabetical order (irrespective of its positional number) before the name of parent alkane. e.g.
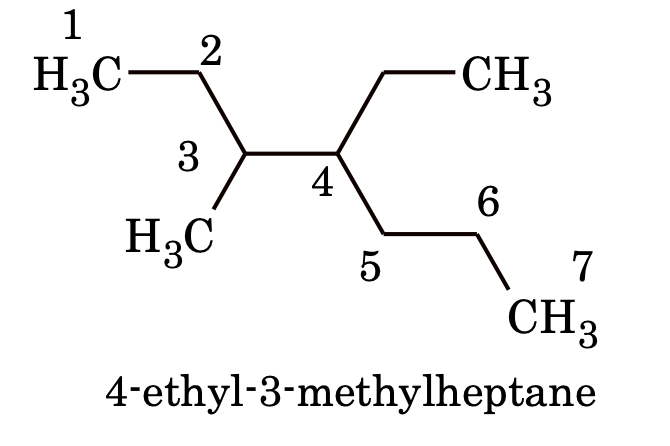
(b) When two different substituents are present at equivalent positions, the numbering of the parent chain is done in such a way that the substituent which comes first in alphabetical order gets the lowest number.
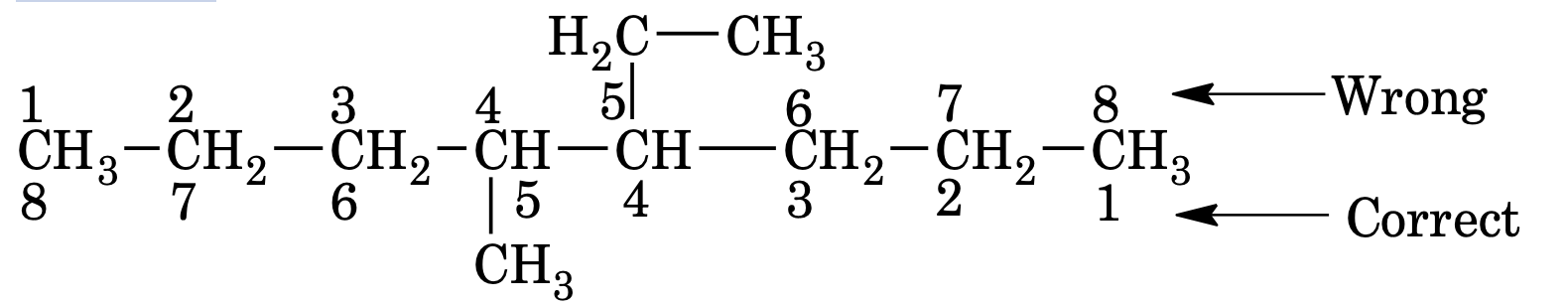
However, the prefixes di, tri etc are not considered while deciding the alphabetical order of the alkyl group.
e.g.
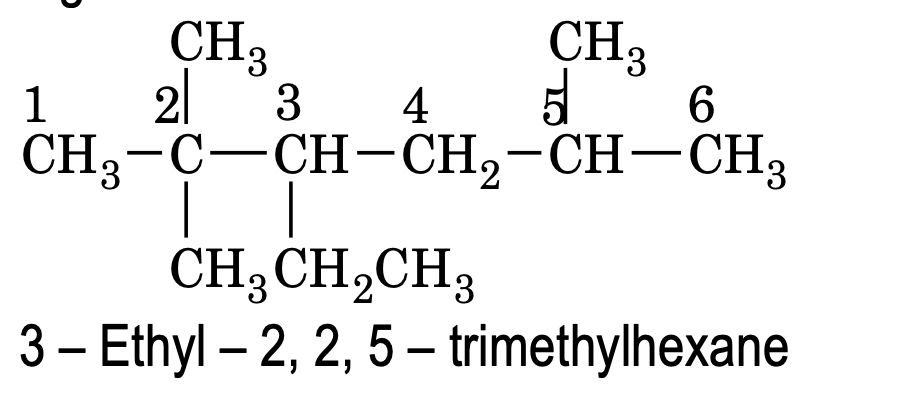
(7) Naming the complex substituent (or substituted substituent)
(a) In case side chain is also branched, it is also numbered from the carbon atom attached to main chain and reported in parenthesis (with in brackets).
e.g.
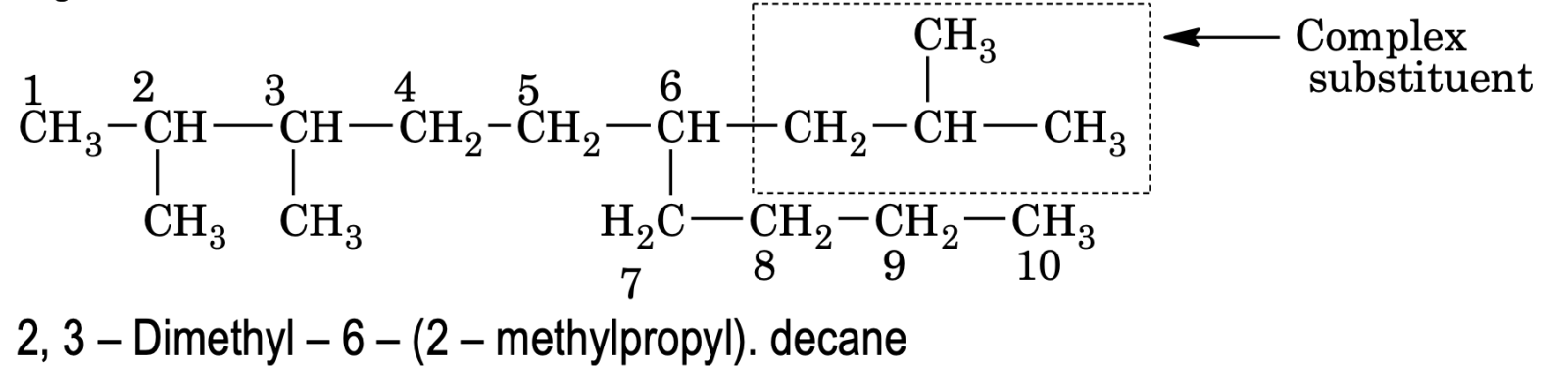
(b) If the chain length of two complex substituents is of equal length, then the complex substituent with larger number of alkyl groups forms a part of the longest carbon chain while the other one is considered the real complex substituent.
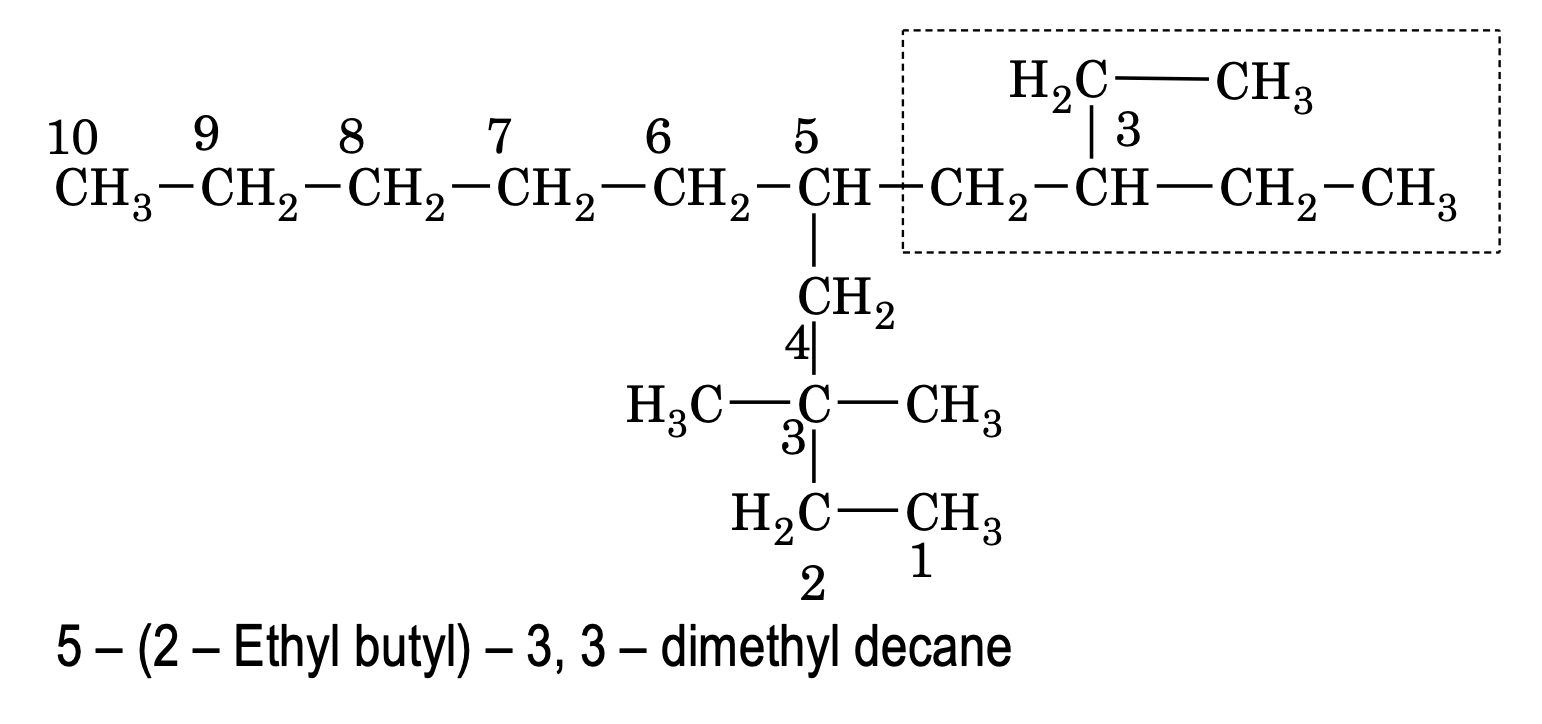
(c) To decide the alphabetical order of the complex substituent its first letter of complete name is considered.
e.g.
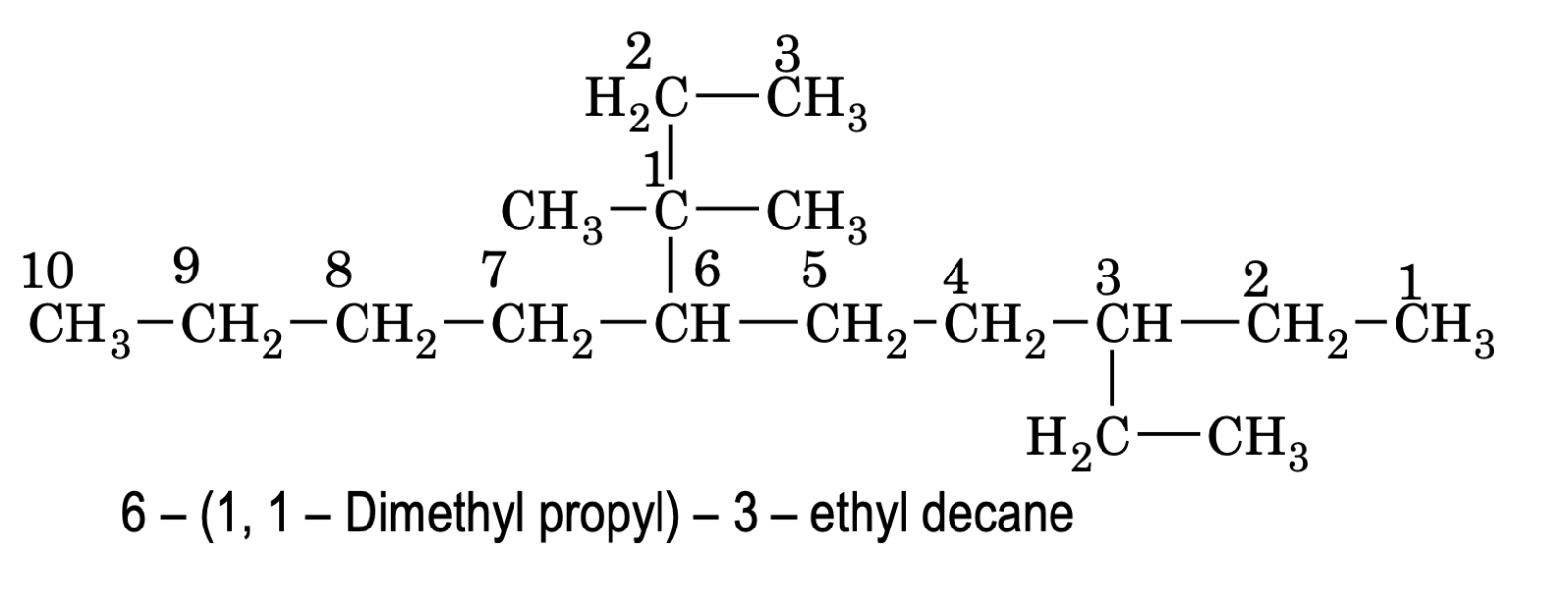
Here ‘d’ of dimethylpropyl is considered for alphabetic order decision and gets priority over ‘e’ of ethyl group.
(d) When the names of two or more substituents are composed of identical words, priority of citation is given to that substituent which has lowest locant at the first cited point of difference within the complex substituent.
e.g.
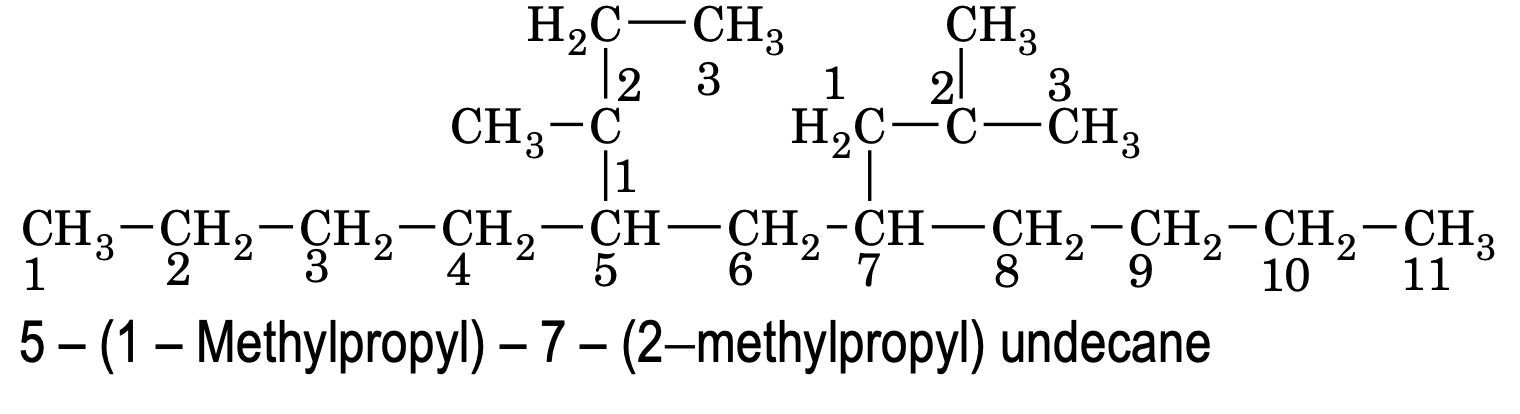
(8) If compound contains similar type of substituted side chain where the use of di, tri prefixes creates confusion. In such cases words like bis (for two), tris (for three), tetrakis (for tour) etc are used to avoid confusion along with locants.
e.g.
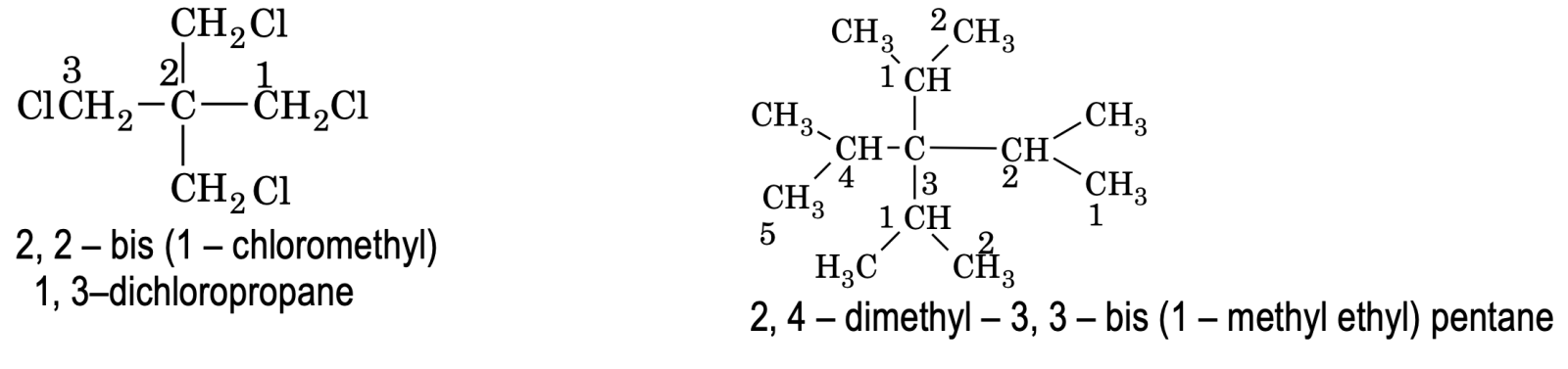
Rules for IUPAC Nomenclature of unsaturated Hydrocarbons
1. The selected parent chain must contain all the multiple bond (if possible) or maximum regardless of the fact that it also denotes the largest continuous chain of carbon or not. But, no other chain containing maximum multiple bonds should be longer than the selected one.
Example
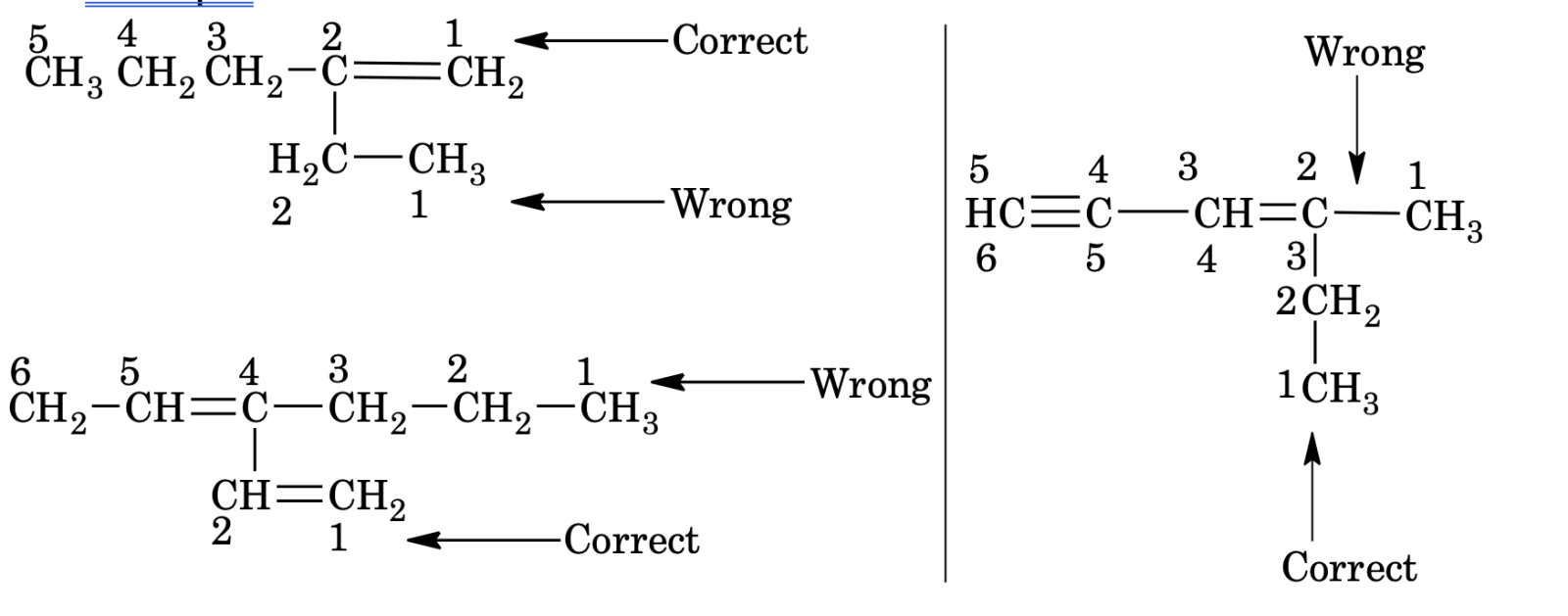
2. Lowest number is assigned to the first unsaturated carbon even if prior rule is violated.
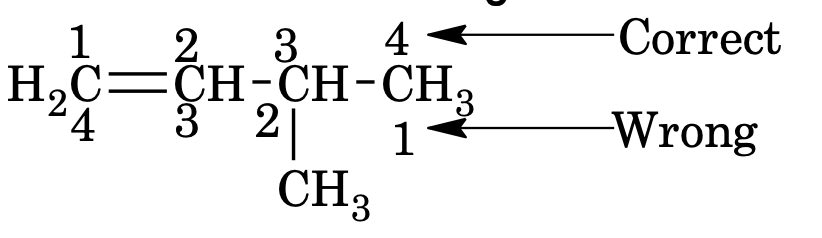
If both double or triple bonds are present, numbering of the chain is done from the end nearer to the double or triple bond
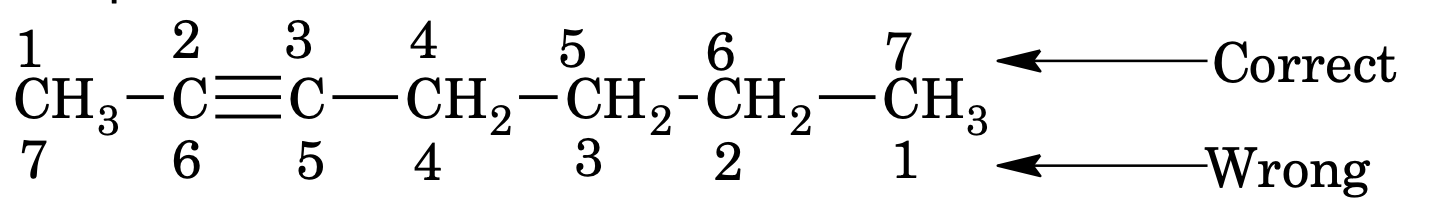
If double and triple bond are present at equivalent position, lowest number is assigned to the double bond
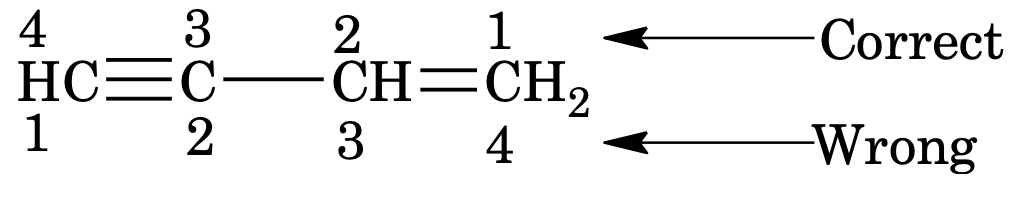
3. In case of unsaturation suffix name of unsaturation is used with hydrocarbon name i.e.
C=C bond ane of hydrocarbon is replaced by – ene
C≡C bond ane of hydrocarbon is replaced by yne
In case of more than one double bond replace ‘ene’ by diene triene etc (for 2, 3 – etc number of double bond) and the parent chain is written as alka–
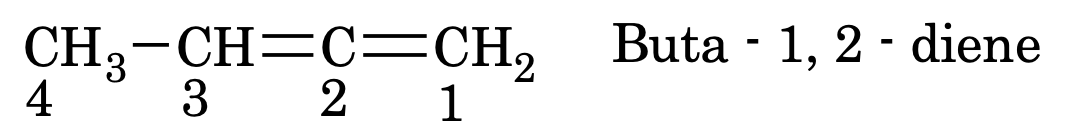
Similarly, for more than one triple bond replace ‘yne’ by adiyne or atriyne etc
4. If both double and triple bonds are present in a compound, (two suffixes are to be used for compound containing both the unsaturation) it is named as Alkenyne.
5. According to latest convention while naming unsaturated hydrocarbons, the locant of (position) of the double bond or triple bond is placed immediately before the suffix ‘ene’ or ‘yne’ and not before the word root as was practiced earlier.

If both double and triple bonds are present in the compound, their locants are written immediately before their respective suffixes. Such unsaturated compounds are always named as derivatives of alkyne rather than alkene.
e.g.
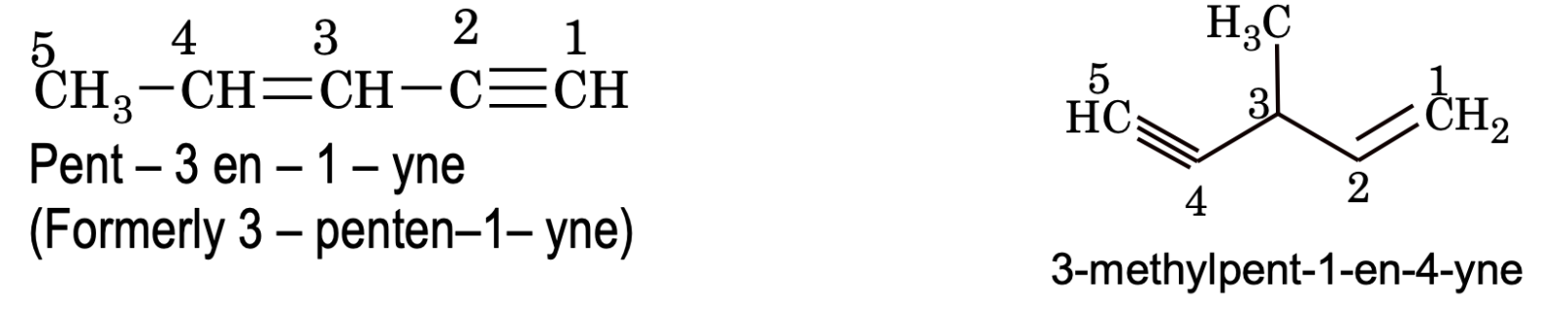
Rules for assigning IUPAC Nomenclature of compounds containing one functional group (along with multiple bonds and substituents)
1. Select the longest possible chain containing the functional group without taking case off weather it is longest chain or not. Again, if the compound contains multiple bonds, the selected chain must contain maximum multiple bonds possible along with functional group even violating the prior rule (i.e. longest chain in compound possible).
e.g.
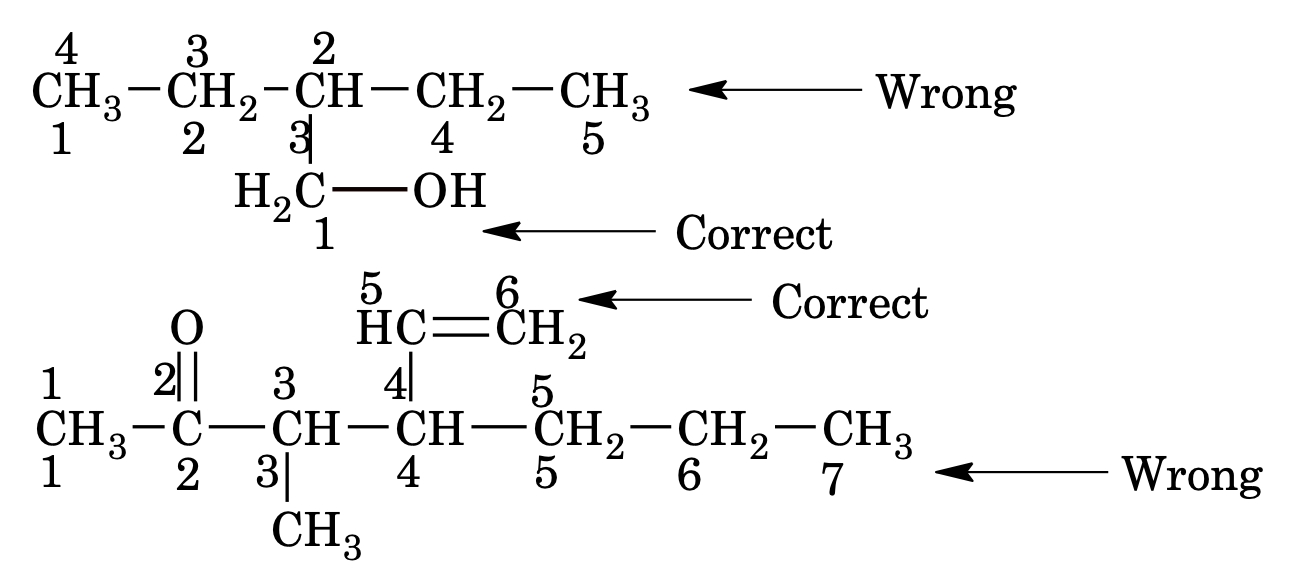
2. The selected parent chain is numbered in such a way that carbon attached to functional group gets the lowest possible number following by double or triple bonds even the rule of lowest sum (or lowest locant) is violated.
The order for numbering a carbon chain follows the sequence
Functional group > unsaturation (Multiple bonds) > substituents and side chain (alkyl groups)
If the functional group contains carbon atom and is attached with parent chain, the carbon atom of functional group is also induced in parent chain (eg. >C=O, -COOH, -CHO etc)
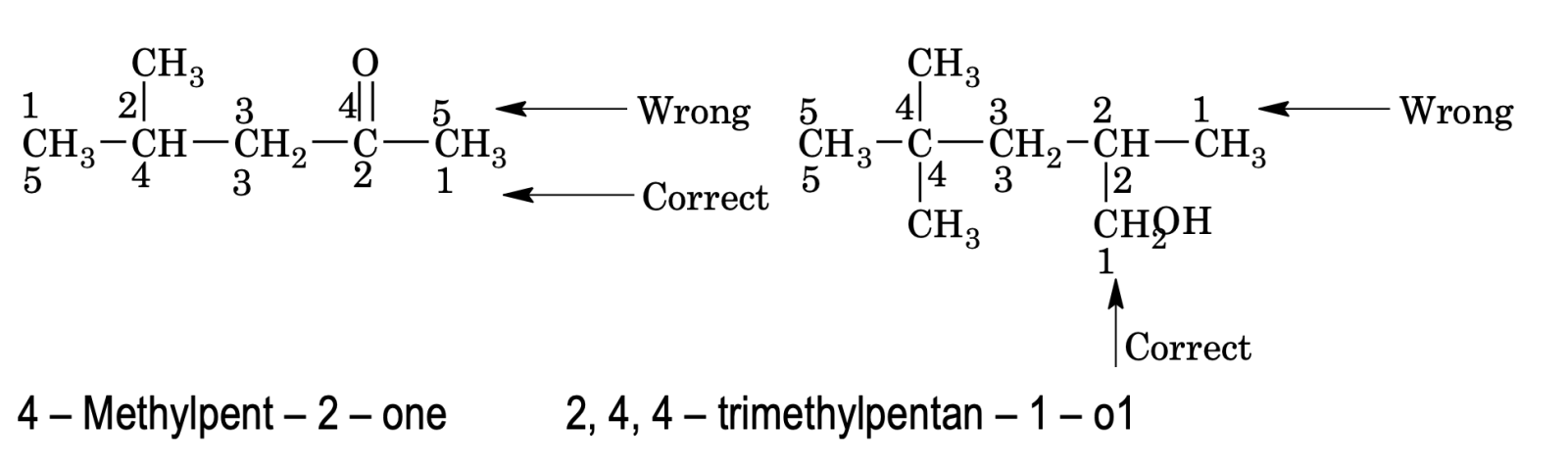
3. When a chain terminating functional group such as –CHO, –COOH, –COOR, – CONH2, –COCl, –CN etc is present, its carbon is included in main chain and is always given number 1, the locant 1 can be omitted for the principal functional group is omitted. The locant number is written before suffix of functional group separated by hyphen (–) sign.
e.g.
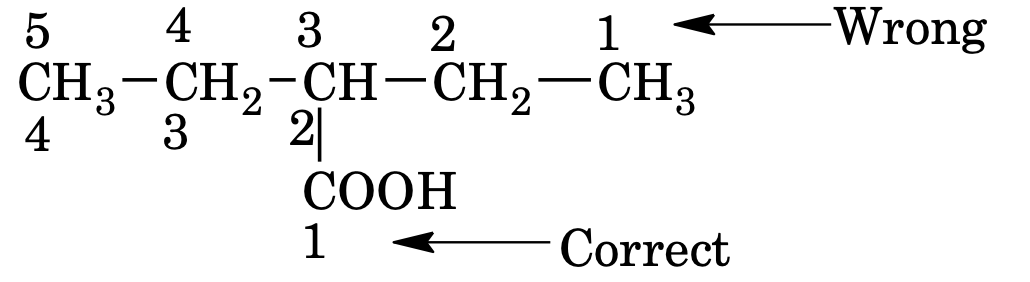
3 – Ethylpropanoic acid
4. If a compound contains two or more like groups, the numerical prefixes di, tri, tetra etc are used and the terminal ‘e’ from the primary suffix is retained while writing its IUPAC Names.
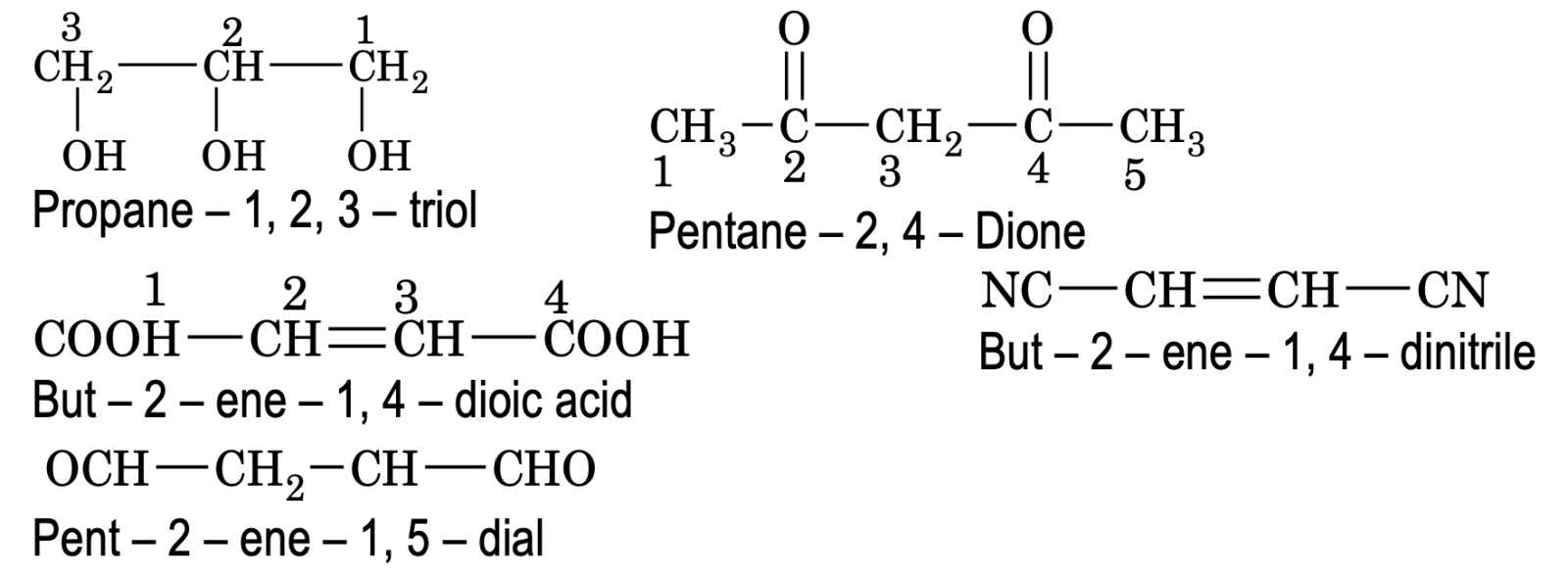
Poly functional compounds containing more than two like functional groups (eg – COOH, – CHO etc)
According to latest convention. If an unbranched carbon chain is directly linked to two like functional group, the organic compound is named as a derivative of the parent alkane which does not include the carbon atoms of the functional group.
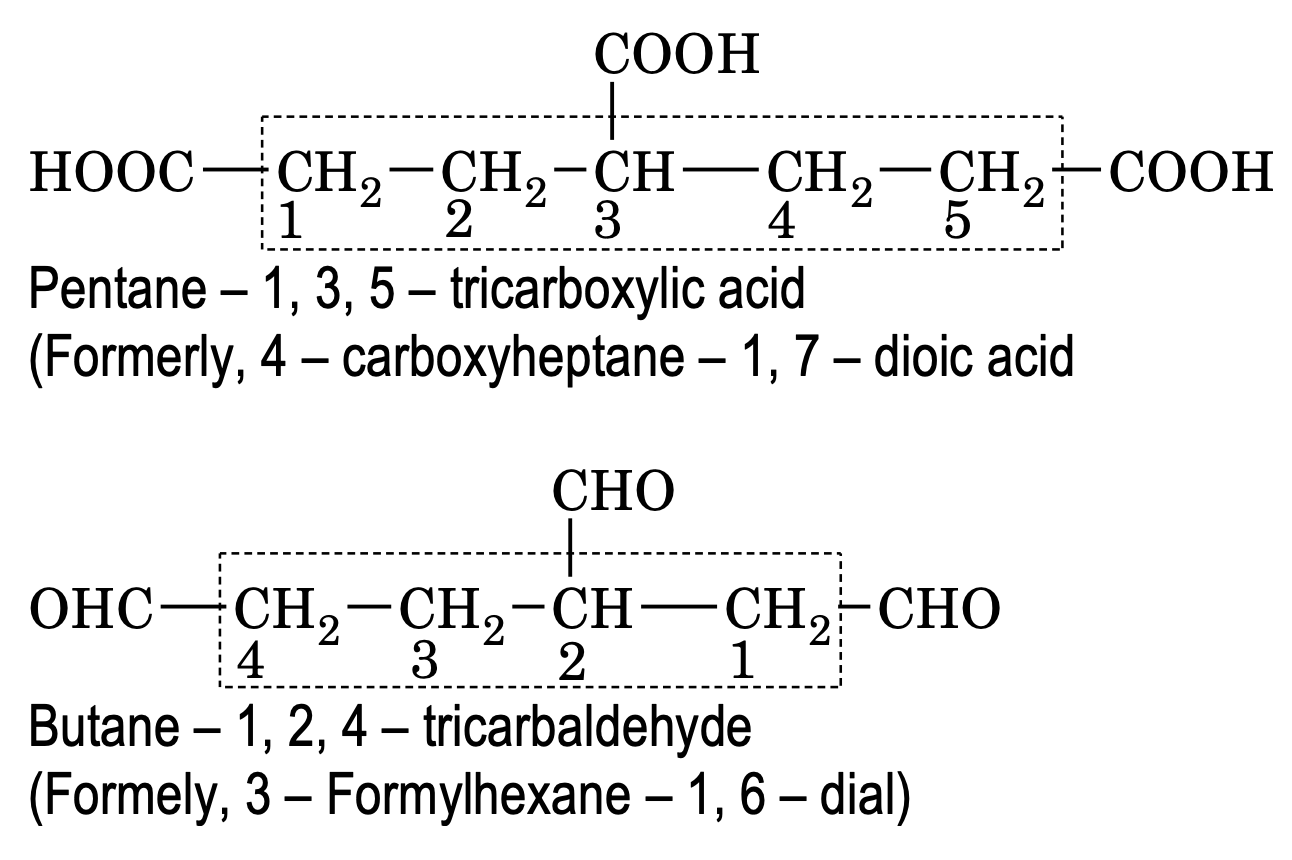
Numbering is done in such a way to give minimum number to the functional group. If a functional group is not directly attached to unbranched chain, then it is considered as substituent while other groups are named as mentioned above.
e.g.
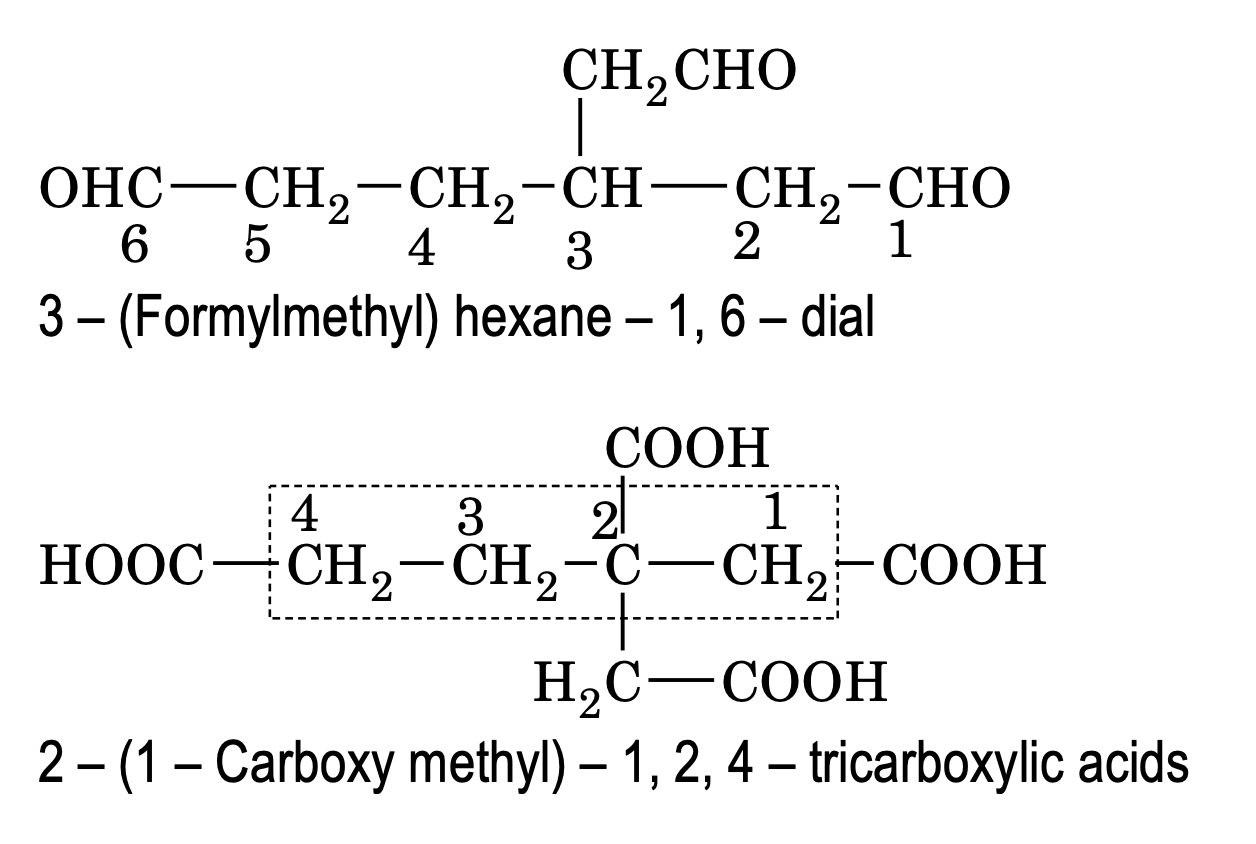
Rules for IUPAC Nomenclature of poly functional compounds:
1. When an organic compound contains two or more different functional groups, one of the functional group is selected as principal (main) functional group while all others functional groups called secondary functional groups and are treated as substituents and arranged in alphabetical order.
The choice of the principal functional group is made on the basis of following order of preference.
Amine salts > carboxylic acids > Sulphonic acids > anhydrides > esters > acid chlorides > acid amides > nitriles > aldehydes > ketones > alcohols > phenols > thiols > amines > ethers > alkenes > alkynes > halogens, alkyl, nitro group, alkoxy
While writing the name of poly functional compounds the principal functional groups is indicated by adding the secondary suffix to the word root while the secondary functional groups are indicated by adding suitable prefixes to the word root (prefixes used are) listed below.
|
Secondary functional group |
Prefix |
Secondary functional group |
Prefix |
|
- X ( F, Cl, Br, I ) |
Halo |
- CN |
Cyano |
|
- OH |
Hydroxy |
R – CH = CH |
Alkenyl |
|
- OR |
Alkoxy |
R – C º C - |
Alkynyl |
|
- SH |
Mercapto |
- CHO |
Formyl or Aldo |
|
- SR - SO3H – Sulpho |
Alkyl thio |
> C = O |
Oxo or keto |
|
- NH2 |
Amino |
- COOH |
Carboxy |
|
- NHR |
Alkyl amino |
- COOR |
Carbalkoxy |
|
- NR2 |
Dialkyl amino |
-COCl Haloalkonyl |
Or Alkoxycarbonyl |
|
= NH Carbonyl |
Imino |
- CONH2 |
Carbonyl carboxamido |
|
– SO3H |
Sulpho |
2. Selection of the principal chain
The selected chain must contain principal functional group and maximum number of secondary functional groups and multiple bonds, if present. e.g.
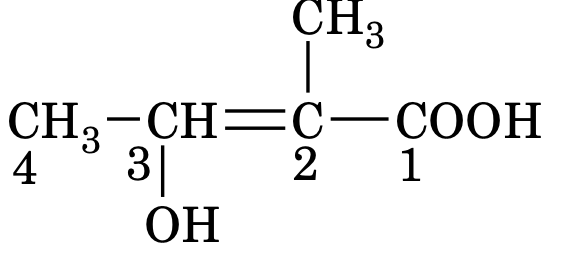
3. Number the chain
The numbering is done in such a way that the main functional group gets the lowest possible number followed by double bond, triple bond and the substituents i.e.
Principal functional group > double bond > triple bond > substituents
4. The prefixes for the secondary functional groups and other substituents are placed in alphabetic order if two group are of same priority than the one which comes first in alphabetic order gets the lower loctant.
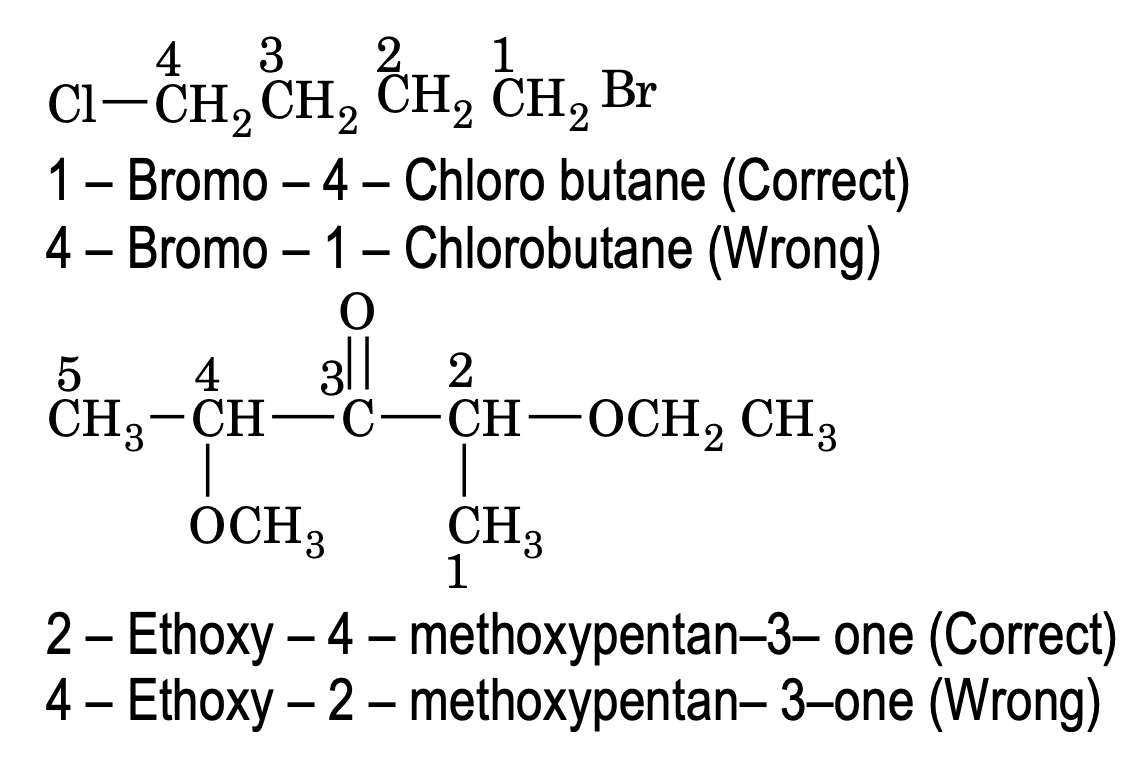
Nomenclature of carboxylic acid derivatives
(a) Acid chlorides
These are named by changing the ending ‘–ic’ acid’ of the corresponding carboxylic acid to‘–yl’ chloride. For example CH3CH2COCl is propanoyl chloride
(b) Esters
These are named by writing first the alkyl group attached to oxygen atom followed by the word derived from carboxylic acid with the replacement of ‘–ic’ acid to ‘–ate’. For eg.
CH3 COOC2H5 is ethyl ethanoate
(c) Acid Anhydrides
These are named by changing the word acid of the parent carboxylic acid to anhydride. For example the compound
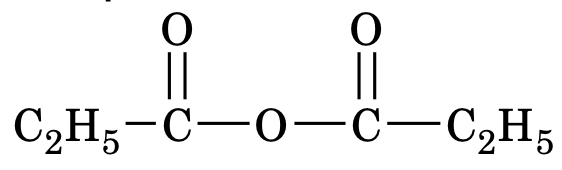
Propanoic anhydride
(d) These are named by replacing the ending – oic acid of the corresponding carboxylic acid with the ending ‘ – amide’. For example – CH3CONH2 is ethanamide
Following are some important changes made in IUPAC recommendation.
(i) Locants (numerals & letters) are placed immediately before the part of the name to which they relate e.g. hex – 2 –ene and not hexene–2.
(ii) The locant 1 (unity) is often omitted when there is no ambiguity e.g. 2– chloroethanol and not 2–chloroethanol – 1.
(iii) Commas are used to separate locants that refer to the same part of name i.e. locants of a series, eg, 1, 2 – dichloroethane.
(iv) Full stops [periods] separate numerical ring size indicators in names, eg, bicyclo (3.2.1) octane.
(v) Hyphens separate or syllables of a name.
(a) Locants from the words, eg – 2 – chloroethanol
(b) adjacent locants referring to different parts of the name but preferably parentheses should be inserted eg N – acetyl – N – (2 – Naphthyl) benzamide.
(c) Stereo descriptor and the name, eg, cis – 2 – butane
Bond – line notation of organic Molecules
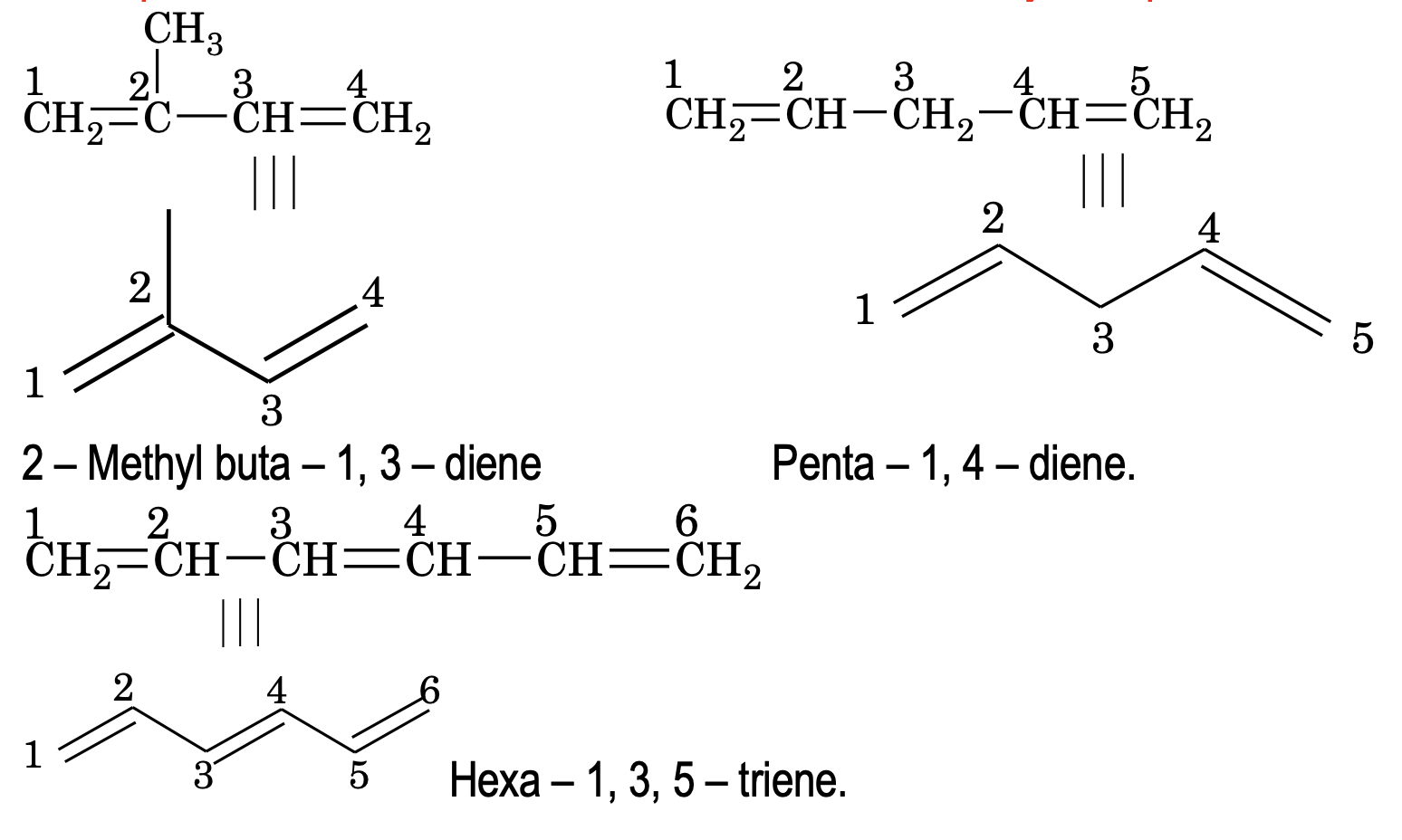
It is simple and convenient method of representing structures. In this notation, bonds are represented by lines and carbon atoms by lines end i.e. intersections. It is assumed that required number of H – atoms are present wherever they are necessary to satisfy the tetracovalency of carbon. For example – 2 – methylbuta – 1, 3 – diene, penta – 1, 4 – diene and hexa – 1 ,3, 5 – triene may be represented as follows.
7. If a compound contains an alicylic ring directly linked to benzene ring, it is named as a derivative of benzene. e.g.
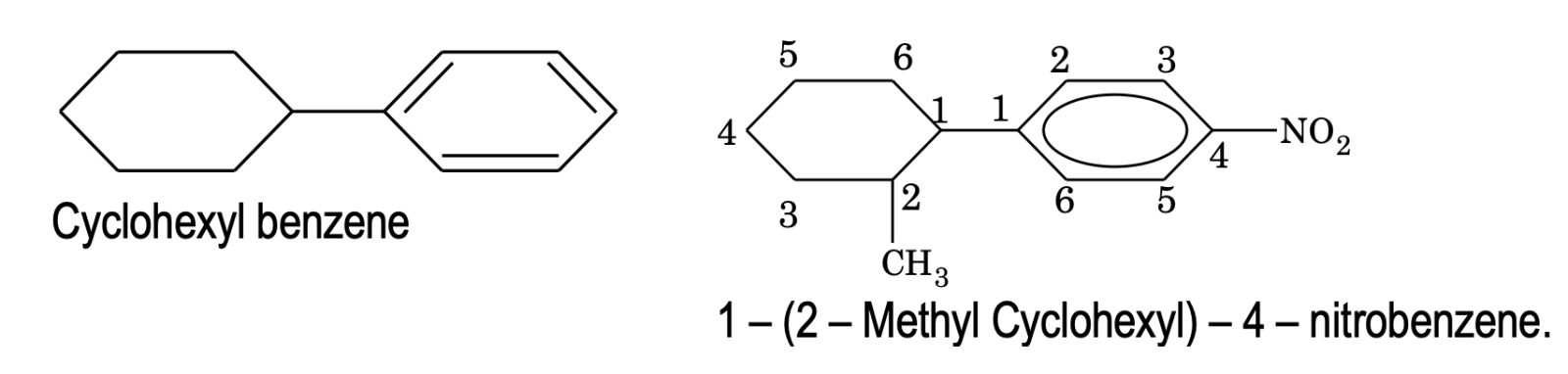
8. If some functional group along with other substituent groups are present in the ring, it is indicated by some appropriate prefix or suffix and its position is indicated by numbering the carbon atoms of the ring in such a way that the functional group gets the lowest number.
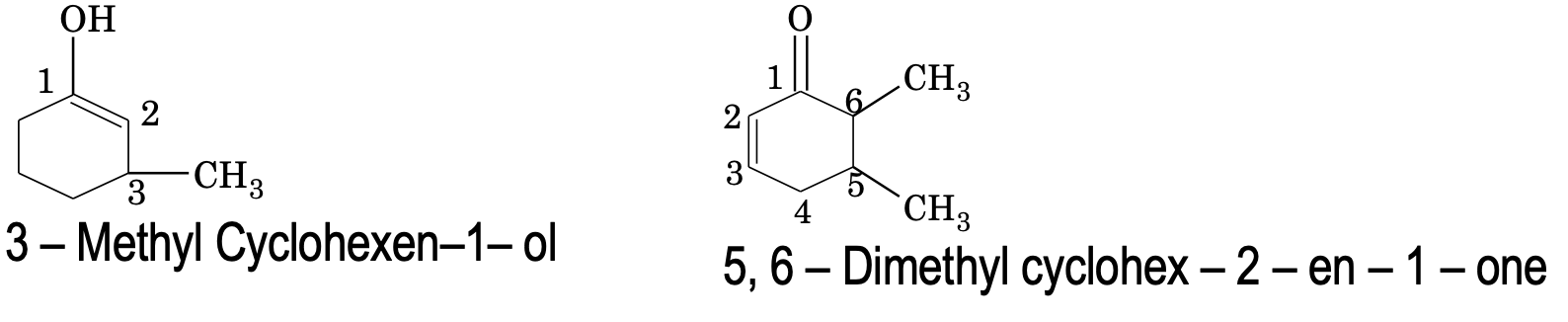
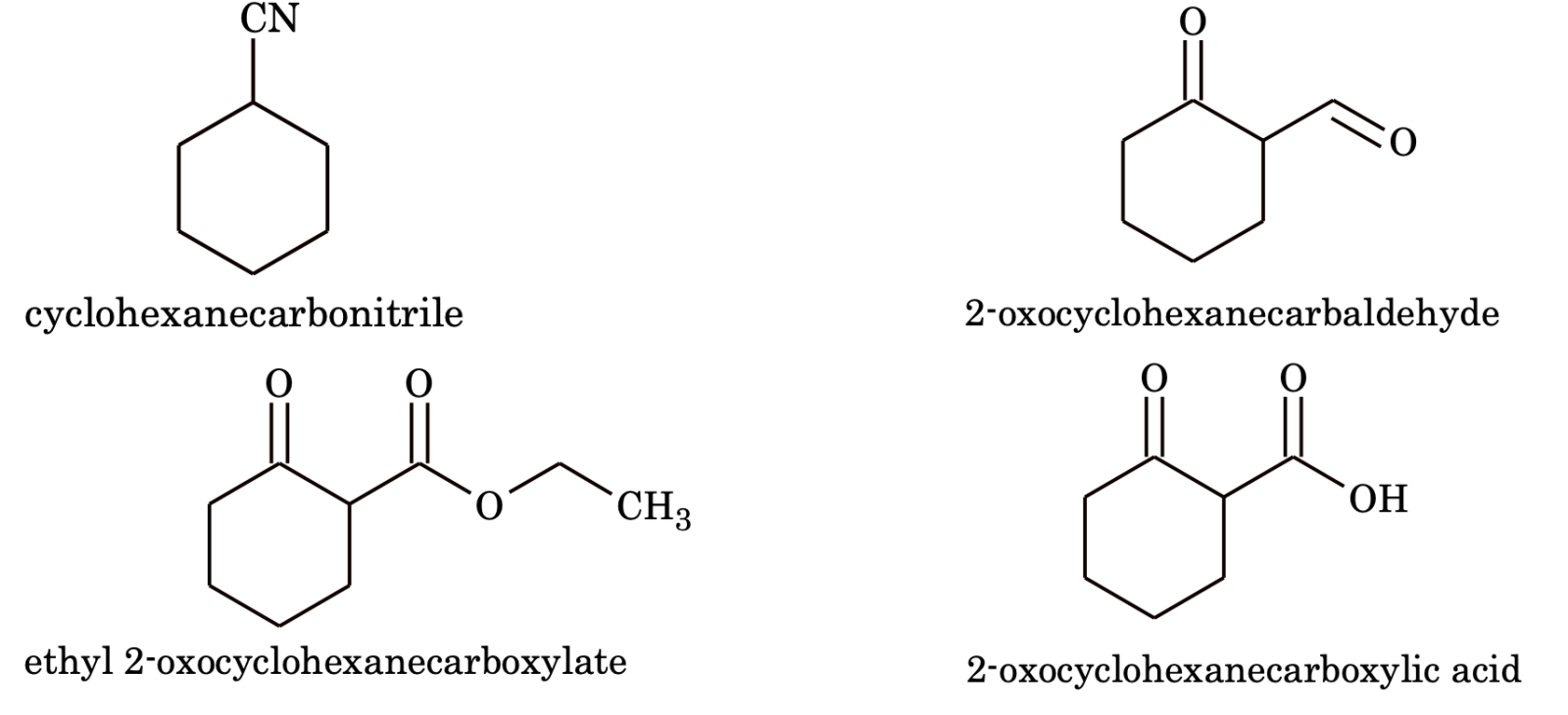
9. If an alicyclic compound contains carbon containing functional groups are not included in the parent name of the alicyclic system. For such, the following prefixes and suffixes for the functional groups are commonly used.
|
Functional group |
Prefix |
Suffix |
|
- CHO |
Formyl |
Carbaldehyde |
|
- COOH |
Carboxy |
Carboxylic acid |
|
- COX (X = Cl, Br, I) |
Halo carbonyl |
Carbonyl halide |
|
- COOR |
Alkoxycarbonyl or Carbalkoxy |
Alkyl carboxylate |
|
- CONH2 |
Carbamoyl |
Carboxamide |
|
- CN |
Cyano |
Carbonitrile |
e.g.
Rules for IUPAC nomenclature of alicyclic compounds
1. A prefix cyclo is added to corresponding straight chain. Hydrocarbon to get the name of alicyclic compounds.
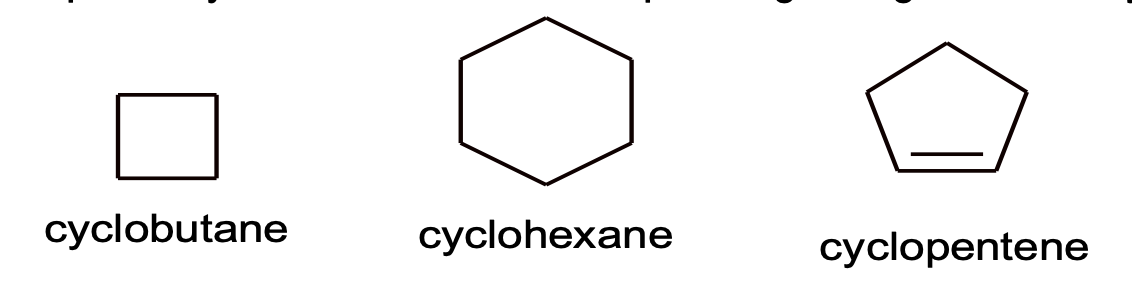
2. If two or more alkyl group or other substituent group are present in the ring, their positions are indicated by Arabic numerals is 1, 2, 3, 4 etc. while numbering the substituent which comes first in alphabetical order gets the lowest number
e.g.
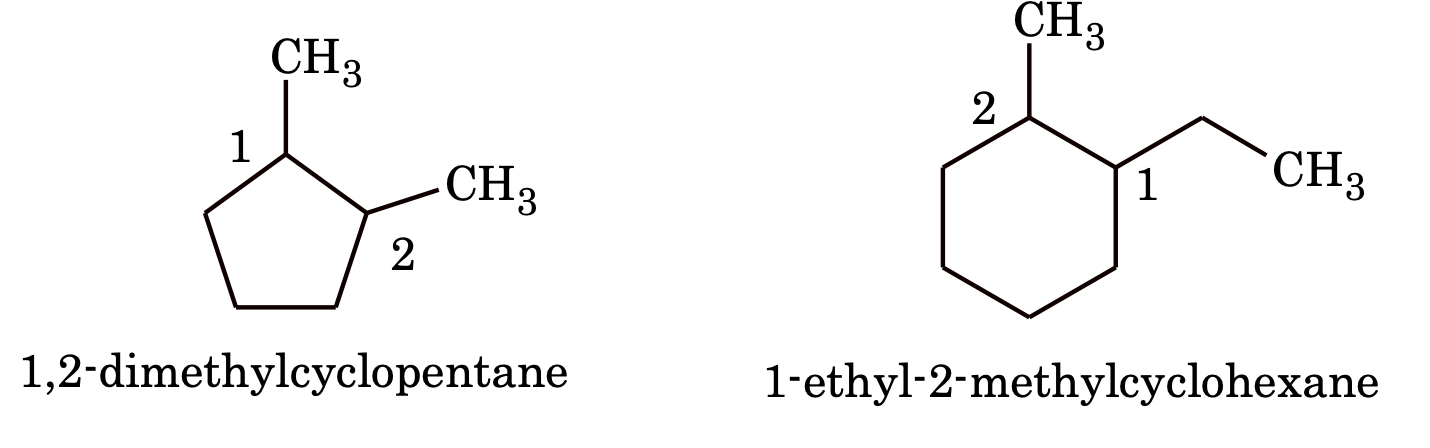
(Parenthesis are used wherever necessary to avoid confusion)
3. (a) If the ring contains more or equal to the number of carbon atoms than the alkyl groups attached to it, it is named as a derivative of Cycloalkanes otherwise it is named as derivative of alkane and the ring is considered as substituent group. e.g.
(b) If however the side chain contains multiple bond or functional group, the alicyclic ring is treated as substituent irrespective of ring size.
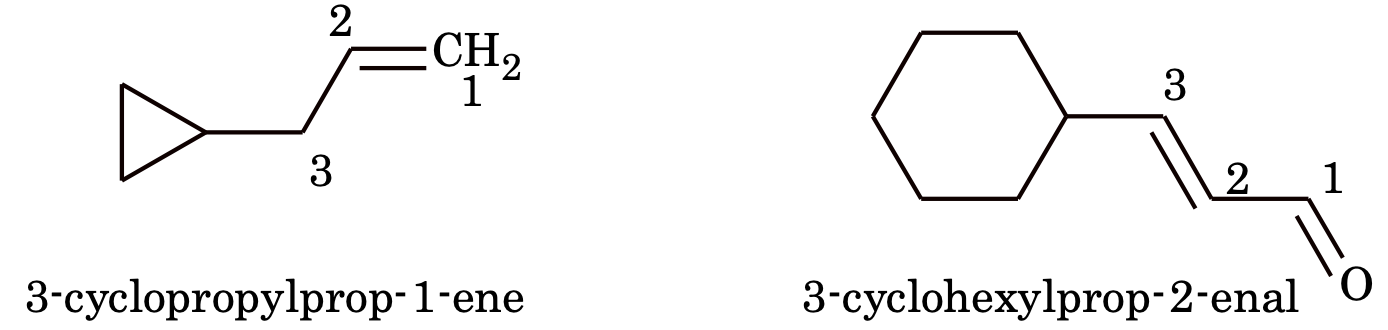
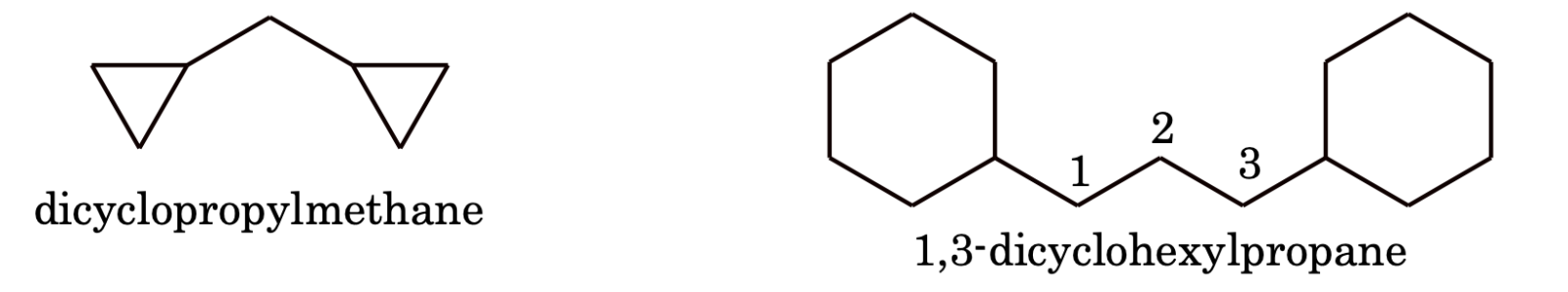
(c) If more than an alicyclic ring is attached to a single chain, the compound is named as a derivative of alkane not taking care of the number of carbon atoms in the ring or chain e.g.
4. If a multiple (double or triple) bond and some substituents are present in the ring, the numbering is done in such a way, that the multiple bond gets the lowest number.
e.g.
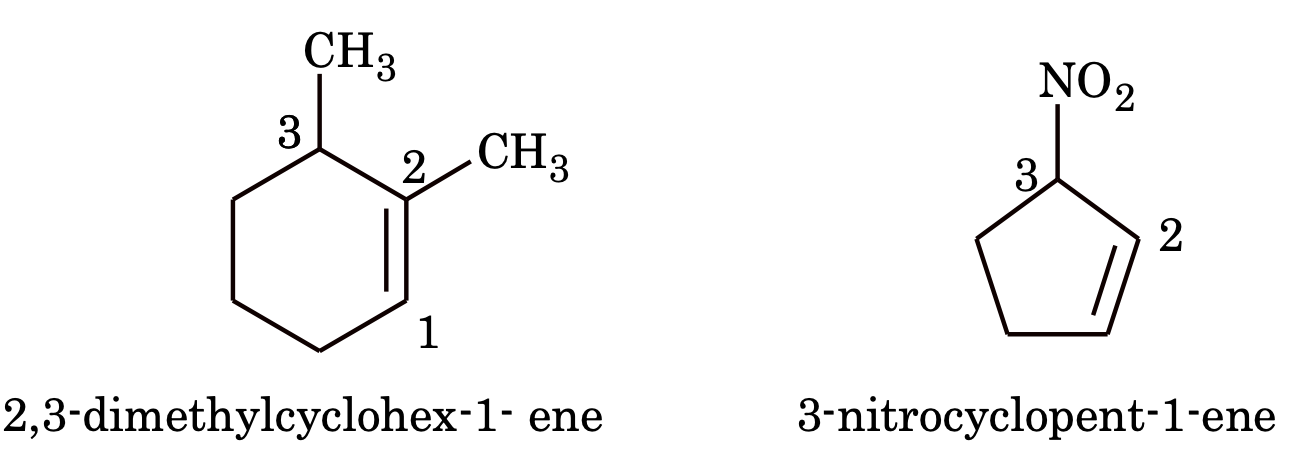
5. If the ring contains a multiple bond and the side chain contains a functional group then the ring is treated as substituent
e.g.
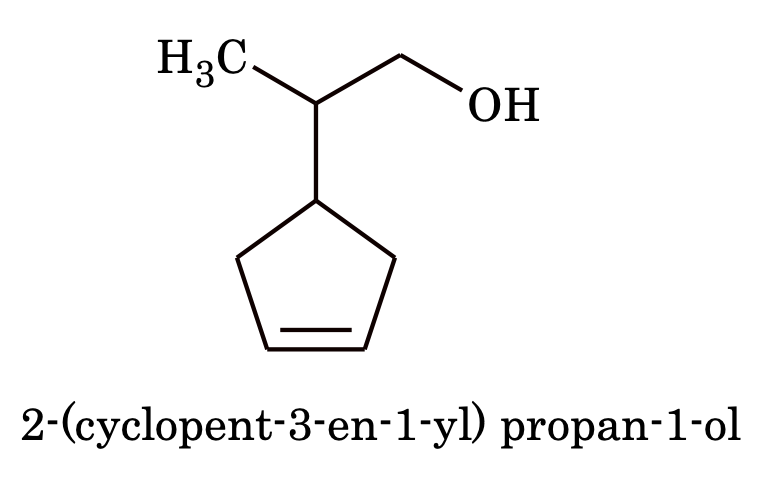
6. If the ring as well the side chain contains a functional group, then either the ring or side chain is considered as substituent depending upon which contains principal functional group e.g.
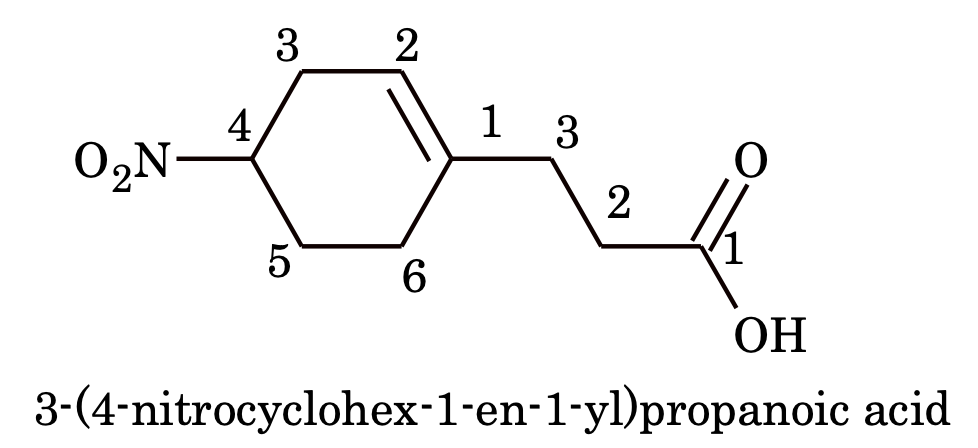
If the ring as well the side chain contains same functional group, then the compound is named as derivative of one, which contains more number of carbon atoms.
NOMENCLATURE OF AROMATIC COMPOUND
The families of aromatic compounds are similar to those of aliphatic compounds with the only difference that each family of aromatic compounds consists of two types of compounds with quit different chemical reactivities
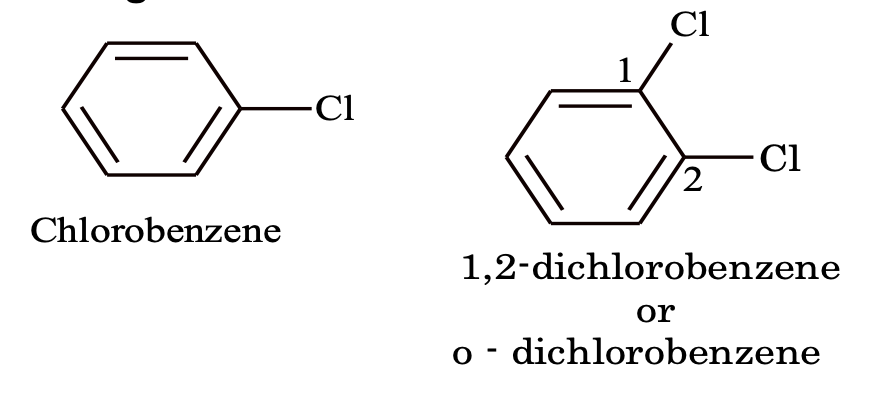
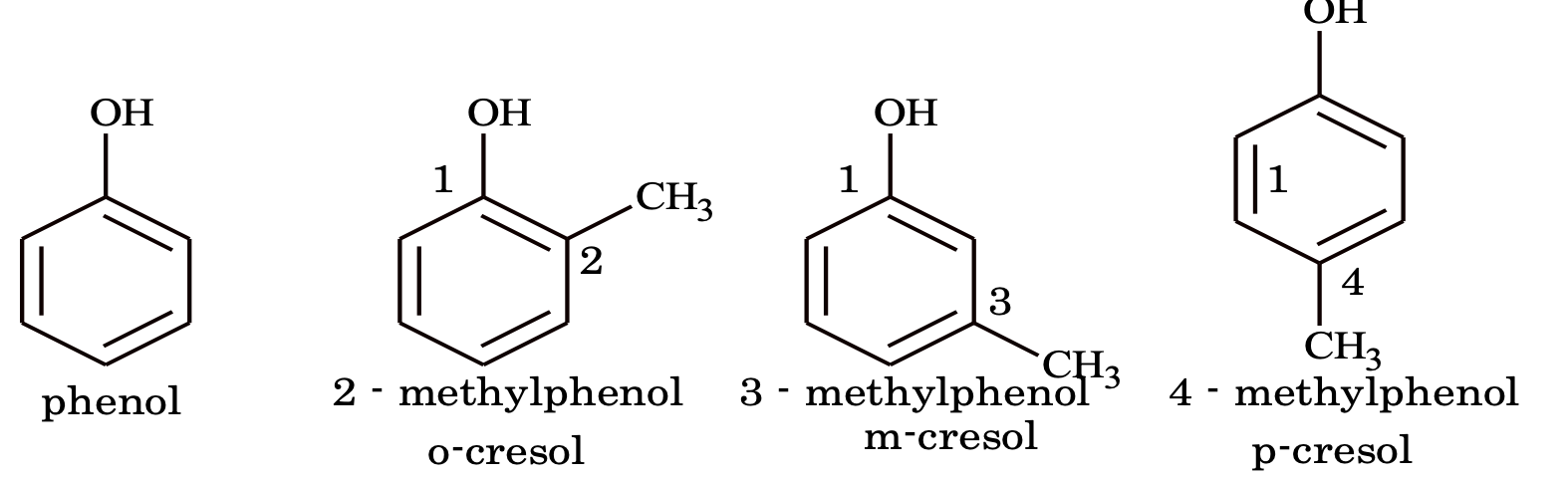
(i) Nuclear substituted: Those in which the fundamental group is directly attached to the benzene ring. Most of these compounds are better known by their common and historical names. In the IUPAC system, they are named as derivatives of benzene. However many of their common names have also been adopted by IUPAC system. The position of substituents in disubstituted benzenes are indicated either by prefixes or by Arabic number such as o–(ortho) for 1, 2, m – (meta) for 1, 3 and p (para) for 1,4.
(ii) Side–chain substituted: Those in which the functional group is present in the side chain of the benzene ring. Both in the common and IUPAC system. These are usually named as phenyl derivatives of the corresponding aliphatic compound (except arene which are named it derivatives of benzene in the IUPAC system. The positions of the substituents on the side chain including the benzene ring are indicated by Greek letter i.e. a, b, g ….. etc in the common system and by Arabic numerals i.e. 1, 2, 3 ……. etc. in the IUPAC system.
(1) Aromatic hydrocarbons (Arenes): Hydrocarbons which contain both aliphatic and aromatic units are called arene. These are of three types
(a) Benzene and alkyl benzenes
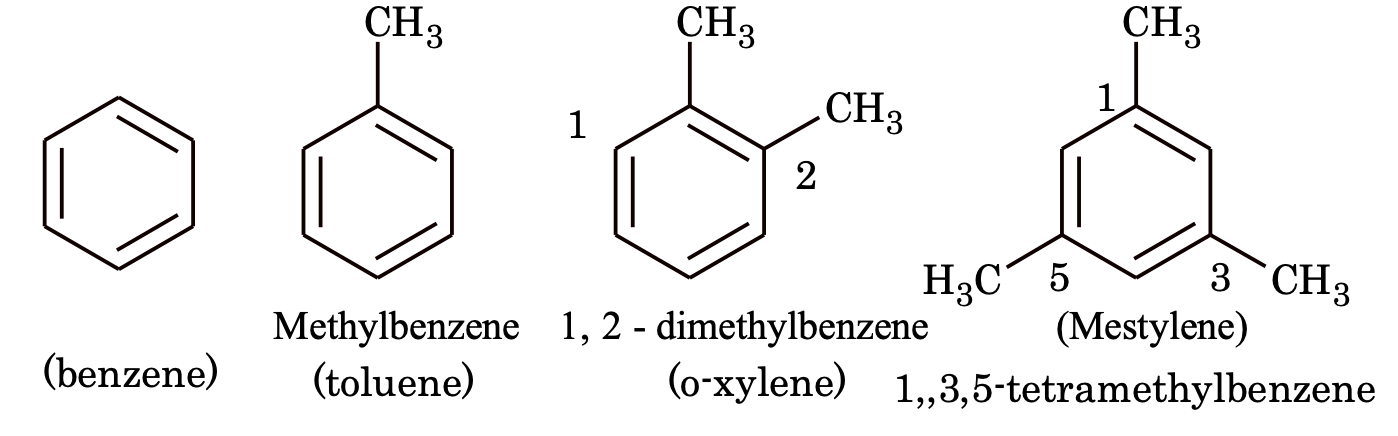
(b) Alkenyl benzenes
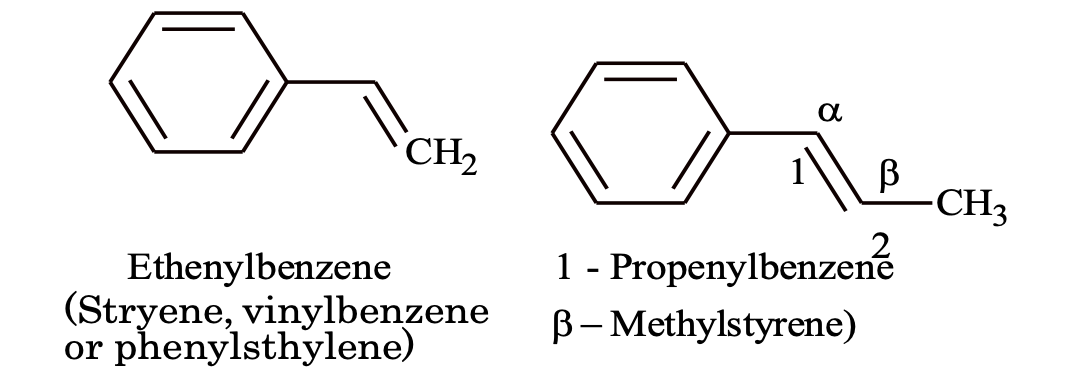
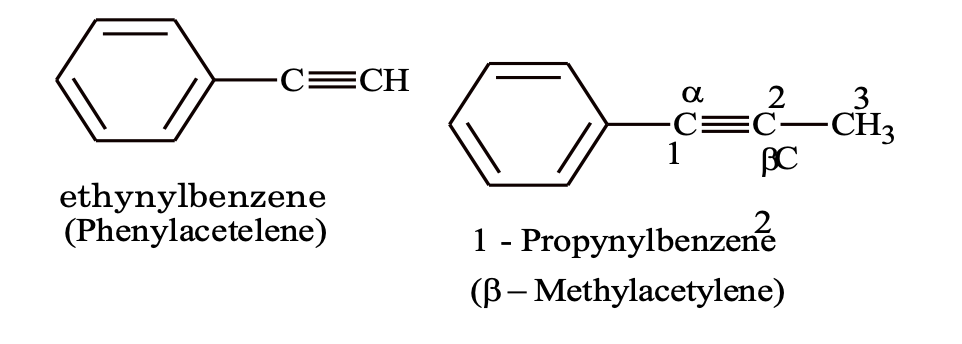
(c) Alkynyl benzene
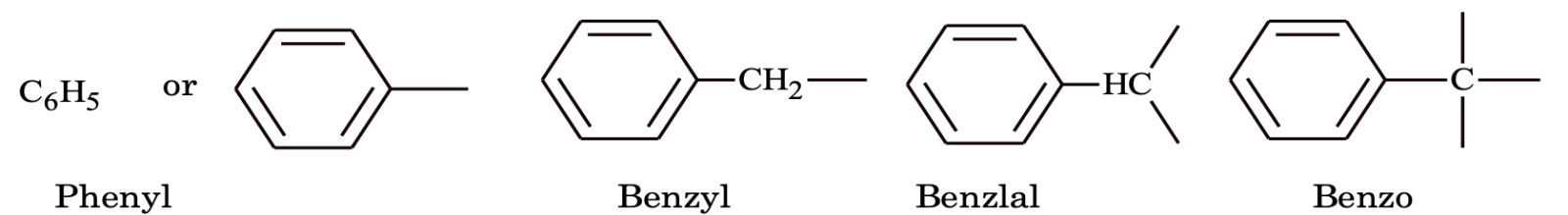
Aryl group
Halogen derivatives
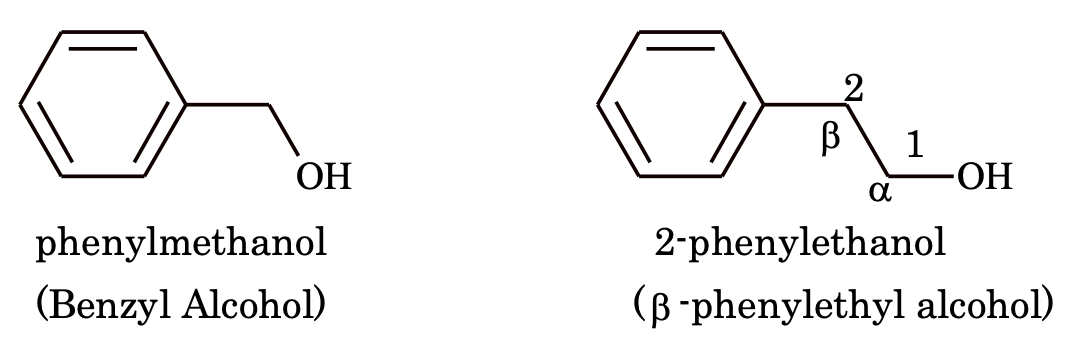
4. Hydroxy derivatives: The nuclear hydroxy derivatives are called phenols while the side chain substituted hydroxy derivatives are called aromatic alcohols.
(i) Phenols – monohydric
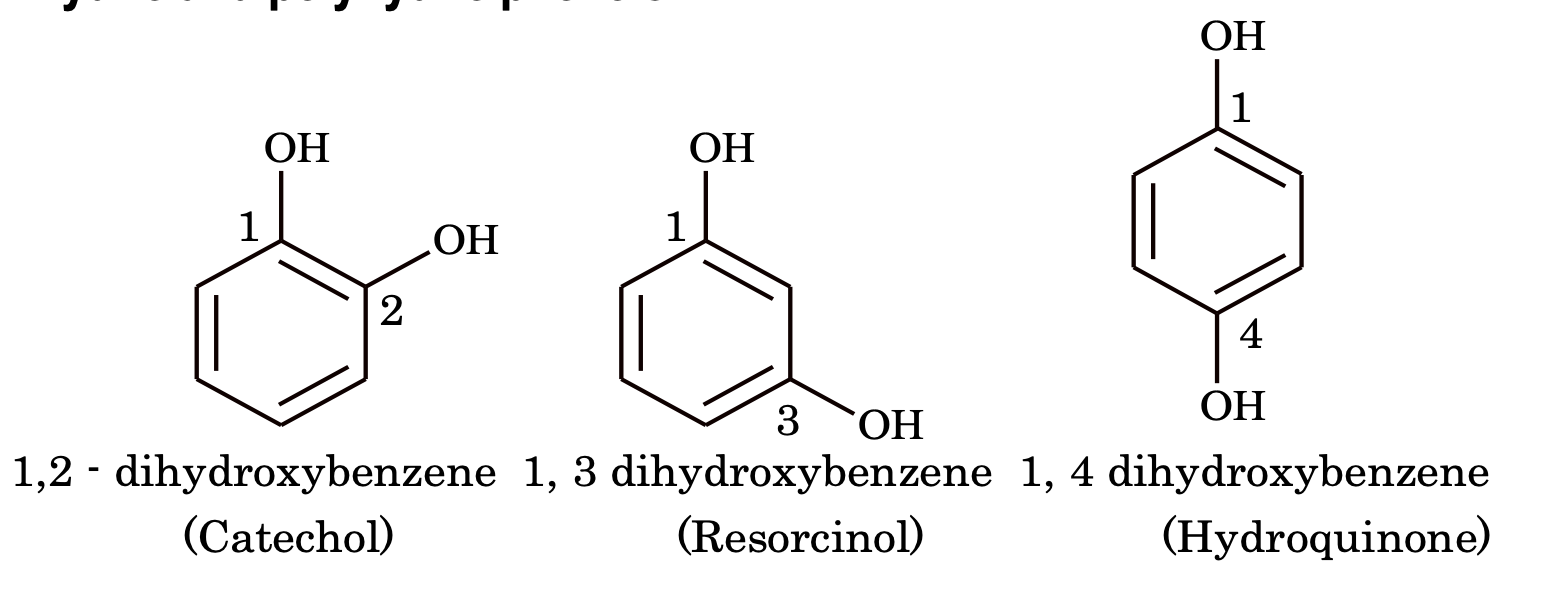
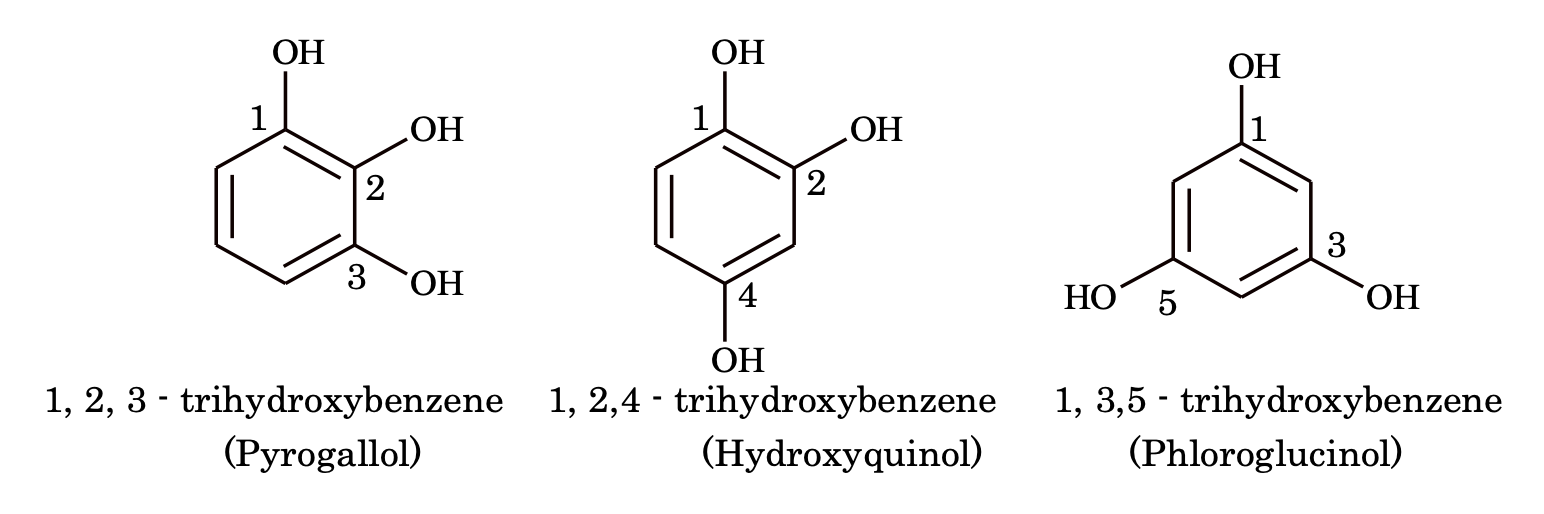
(ii) Dihydric and polyhydric phenols
(iii) Aromatic alcohols
5. Aromatic ether
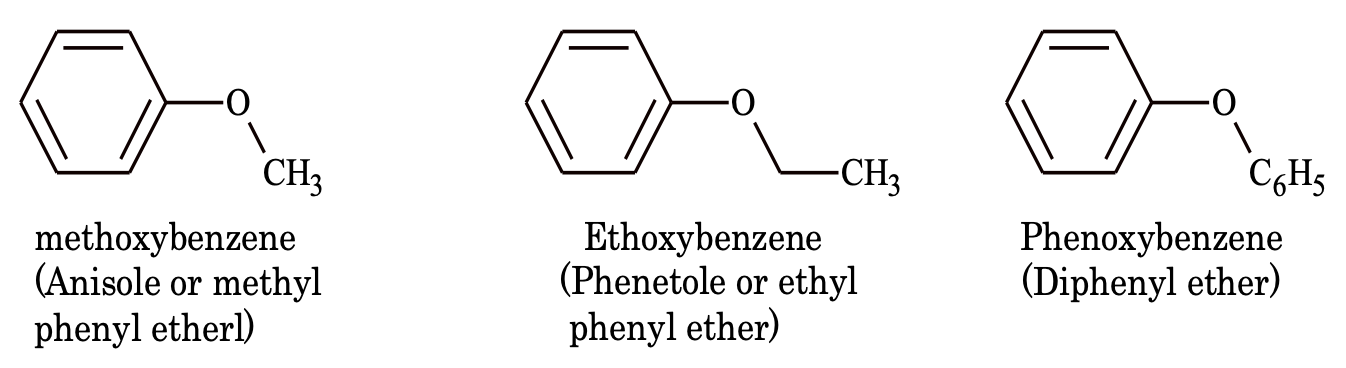
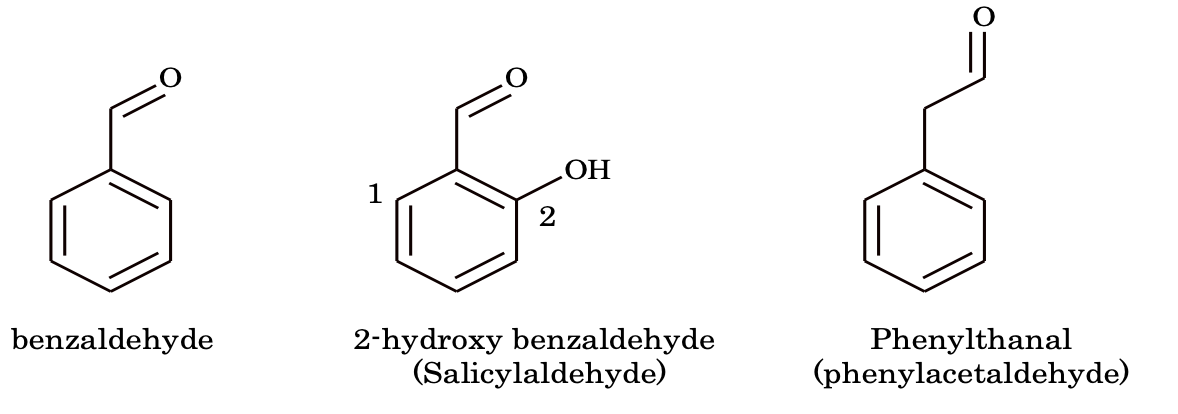
6. Aldehydes
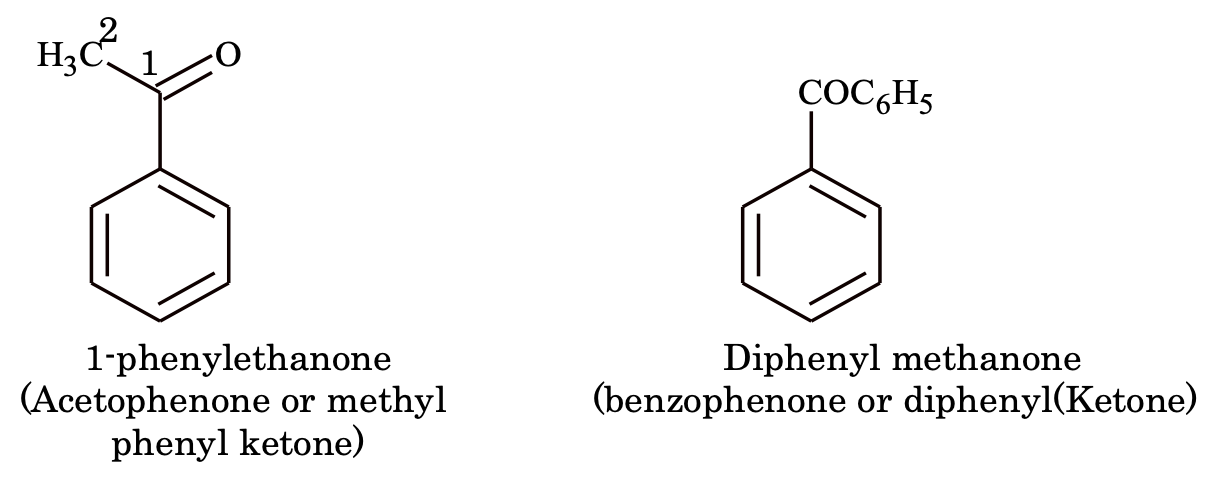
7. Ketones
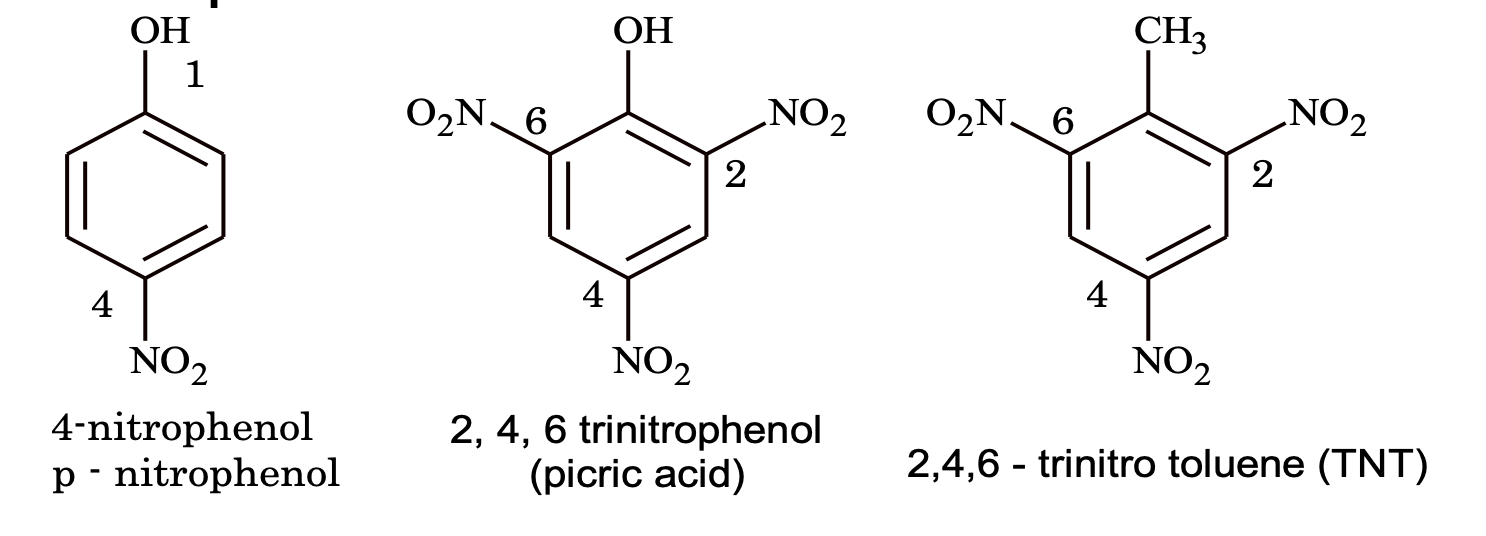
8. Nitro compounds
systematic nomenclature for Di – and polyfunctional aromatic compounds
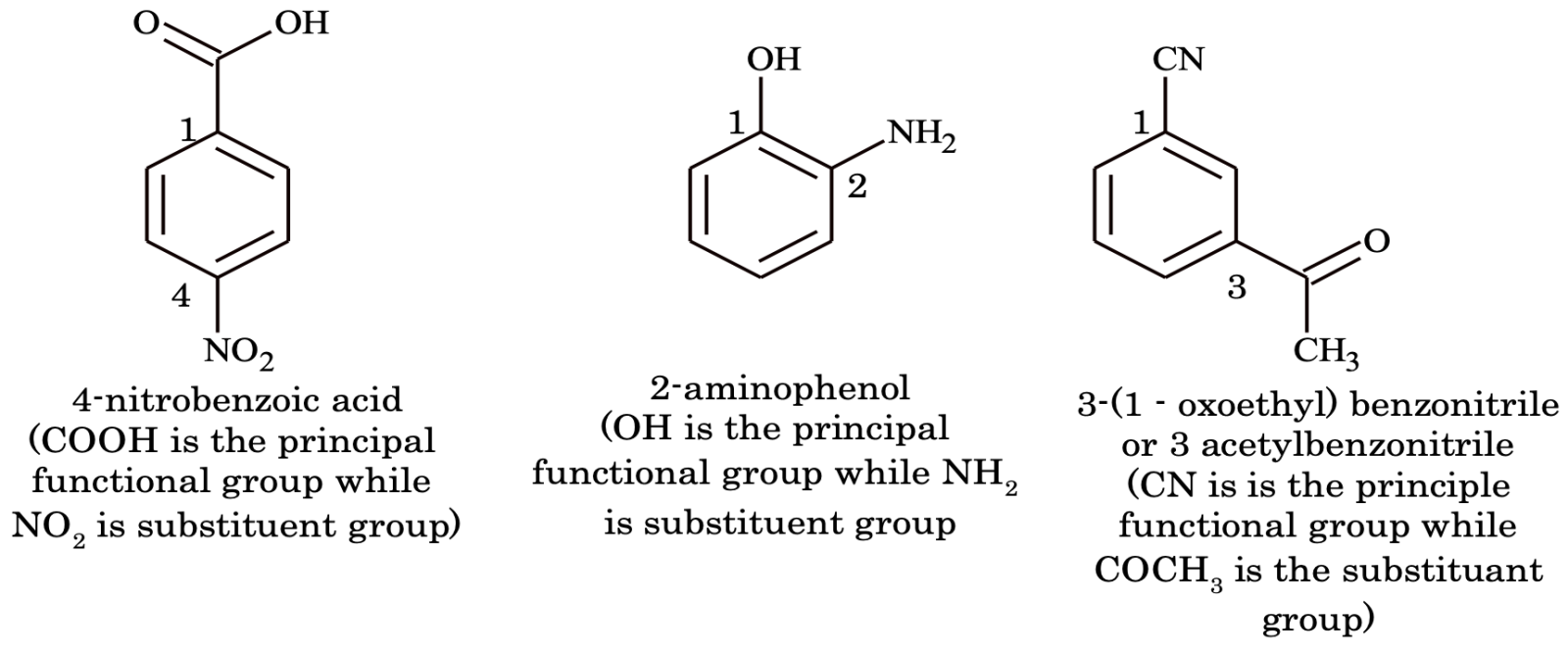
(i) When an aromatic compound contains two or more functional groups. It is named as derivative of the compound with the principal functional group at position 1. e.g.
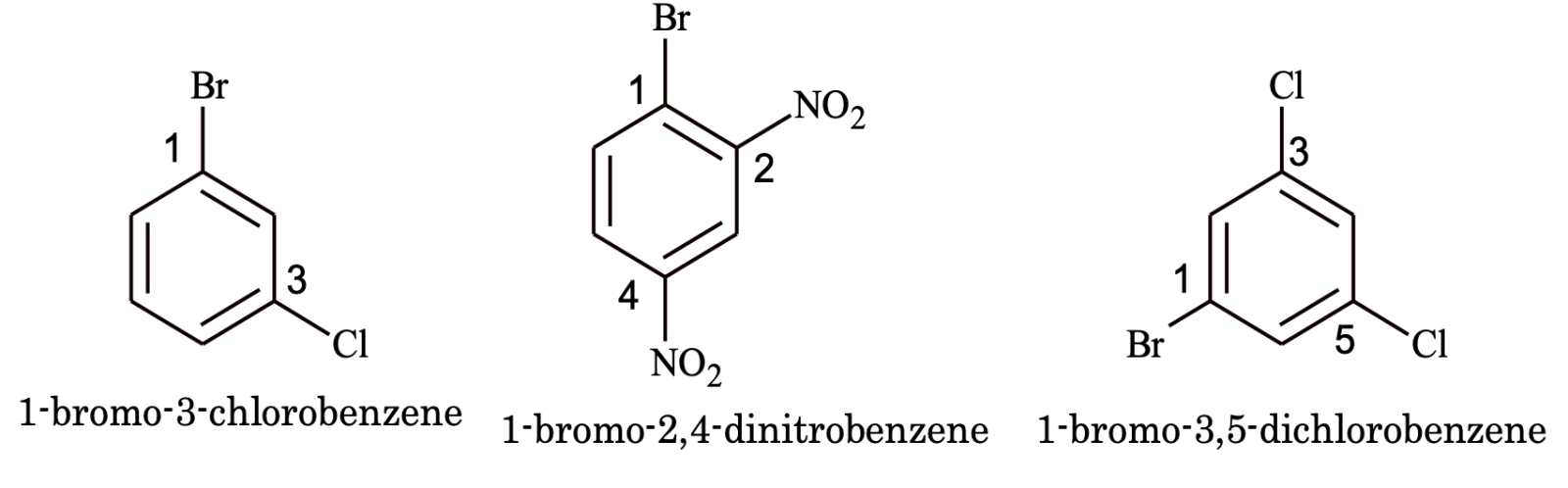
(ii) If all the functional groups present in benzene ring are such which are normally treated its substituent group. The various groups are arranged in alphabetical order with Locant rule for the substituents.
e.g.
(iii) When a substituent is such which when taken together with the benzene ring gives special name of the molecules, then it is named as a derivative of that molecule with the substituent as position of e.g.
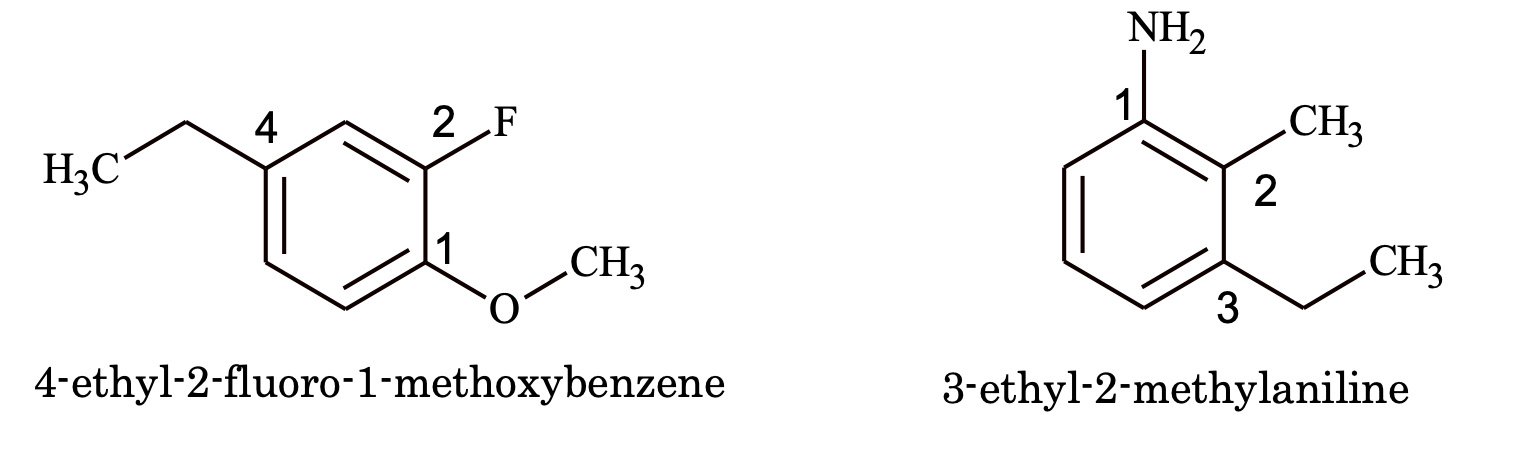
(iv) When of benzene ring is attached to in aliphatic chain having a functional group, it is named as phenyl derivative of that aliphatic compound e.g.

Important Points
- Organic compounds have been classified on the basis of carbon skeleton (structure) or functional group or the concept of homology.
- Acyclic or open-chain compounds (Aliphatic compounds) may be straight or branched chains.
- Cyclic or closed-chain compounds (Ring compounds) contain one or more closed-chains or rings of atoms in their molecules.
- Homocyclic or carbocyclic compounds contain a ring or rings of carbon atom only in the molecules.
- Alicyclic compounds (Cycloalkanes) are carbocyclic compounds which resemble aliphatic compounds in most of their properties. Examples are cyclopropane, cyclobutane, cyclopentane and cyclohexane etc.
- Aromatic compounds consist of at least one benzene ring, i.e., a six-membered carbocyclic ring having alternate single and double bonds.
- Heterocyclic compounds are cyclic compounds containing one or more hetero atoms (O, N, S, etc.) in the ring. Example are furan, thiophene, pyrrole, pyridine, indole and quinoline, etc.
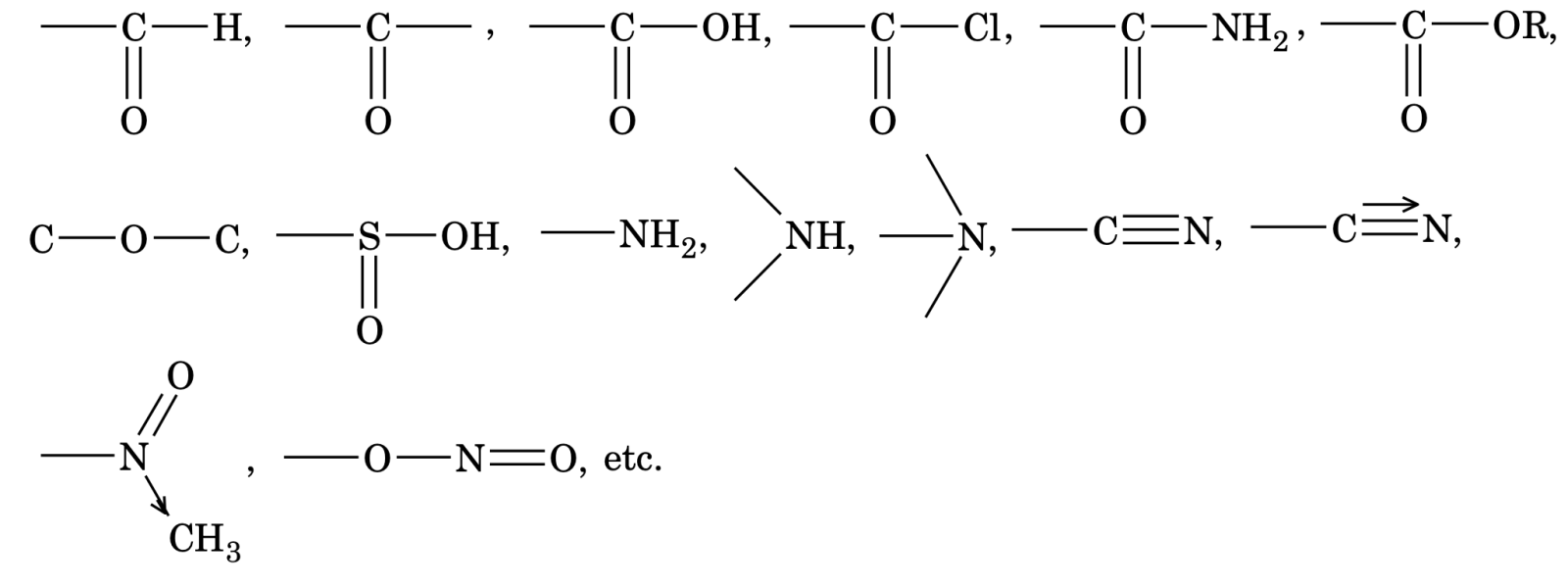
- Functional group is an atom or group of atoms present in a molecule which largely determines its chemical properties. For example, C=C, CºC, X(Cl, Br, I), -OH, -SH,
- Nomenclature of aliphatic compounds having poly functional groups. The order of preference for the choice of principal functional group is Carboxylic acids > sulphonic acids > acid anhydrides > esters > acid chlorides > acid amides > cyanides > aldehydes > Keotnes > alcohols, phenols, thiols > amines > ethers > alkenes, alkynes > halo, nitro, alkyl.