Biotechnology deals with techniques of using live organisms or enzymes from organisms to produce products and processes useful to humans. In this sense, making curd, bread or wine, which is all microbe-mediated processes, could also be thought as a form of biotechnology. However, it is used in a restricted sense today, to refer to such of those processes which use genetically modified organisms to achieve the same on a larger scale. Further, many other processes/techniques are also included under biotechnology.
Importance Of Biotechnology
- It offers a rational approach to under-standing the molecular basis of a number of diseases (e.g., familial hypercholesterolemia, sickle cell disease, the thalassemias, cystic fibrosis, muscular dystrophy).
- Using recombinant DNA technology, human proteins can be produced in abundance for therapy (e.g., insulin, growth hormone, plasminogen activator).
- Proteins for vaccines (e.g., hepatitis B) and for diagnostic tests (e.g., AIDS test) can be obtained
- Recombinant DNA technology is used to diagnose existing diseases and predict the risk of developing a given disease.
- Special techniques have led to remarkable advances in forensic medicine.
- Gene therapy for diseases like sickle cell disease, the thalassemias, adenosine deaminase deficiency (ADA deficiency), and other diseases may be devised.
- In vitro fertilisation leading to a ‘test-tube’ baby, synthesising a gene and using it, developing a DNA vaccine or correcting a defective gene, are all part of biotechnology.
Definition Of Biotechnology
The European Federation of Biotechnology (EFB) has given a definition of biotechnology that encompasses both traditional view and modern molecular biotechnology. The definition given by EFB is as follows:
Biotechnology can be defined as ‘the integration of natural science and organisms, cells, parts thereof, and molecular analogues for products and services’
Principles Of Biotechnology
Among many, the two core techniques that enabled birth of modern biotechnology are:
(i) Genetic engineering:Genetic engineering is the ability to isolate a gene coding for desired product, alter the chemistry of the gene and to introduce it to a host organism in order to change the phenotype of the host organism. This has opened the way either to the more effective production of useful proteins or to the introduction of novel characteristic in the host organism.
(ii) Maintaining a microbial contamination free (sterile) ambience in chemical engineering which will enable the formation of only desired microbe/cells in huge number for the manufacture of biotechnological products like hormones, vaccine, blood clotting factors, antibiotics, enzymes etc. It is necessary to have complete aseptic conditions.
Significance of RDT Over Sexual Reproduction
Sexual reproduction provides opportunities for variations and formulation of unique combinations of genetic setup, some of which may be beneficial to the organism as well as the population. Asexual reproduction preserves the genetic information, while sexual reproduction permits variation. Traditional hybridisation procedures used in plant and animal breeding, very often lead to inclusion and multiplication of undesirable genes along with the desired genes.
The techniques of genetic engineering which include creation of recombinant DNA, use of gene cloning and gene transfer, overcome this limitation and allow us to isolate and introduce only one or a set of desirable genes without introducing undesirable genes into the target organism.
When a piece of DNA, which is somehow transferred into an alien organism, most likely, would not be able to multiply itself in the progeny cells of the organism. But, when it gets integrated into the genome of the recipient, it may multiply and be inherited along with the host DNA. This is because the alien piece of DNA has become part of a chromosome, which has the ability to replicate.
In a chromosome there is a specific DNA sequence called the origin of replication, which is responsible for initiating replication. Therefore, for the multiplication of any alien piece of DNA in an organism it needs to be a part of a chromosome(s) which has a specific sequence known as ‘origin of replication’. Thus, an alien DNA is linked with the origin of replication, so that, this alien piece of DNA can replicate and multiply itself in the host organism. This can also be called as cloning or making multiple identical copies of any template DNA.
Hence Genetic Engineering is the artificial manipulation of the genetic material of organisms, including the creation of novel genetic material (i.e., novel nucleotide sequences). This manipulation occurs to a large extent external to organisms. Genetic engineering is employed to
- Make recombinant DNA
- To purposefully change nucleotide sequences
- To clone DNA
Cloning of DNA from any organism entails five general procedures:
- Cutting DNA at precise locations - Sequence-specific endonucleases (restriction endonucleases) provide the necessary molecular scissors.
- Selecting a small molecule of DNA capable of self-replication - These DNAs are called cloning vectors (a vector is a delivery agent). They are typically plasmids or viral DNAs.
- Joining two DNA fragments covalently - The enzyme DNA ligase links the cloning vector and DNA to be cloned. Composite DNA molecules Comprising covalently linked segments from two or more sources are called recombinant DNAs.
- Moving recombinant DNA from the test tube to a host cell that will provide the enzymatic machinery for DNA replication.
- Selecting or identifying host cells that contain recombinant DNA.
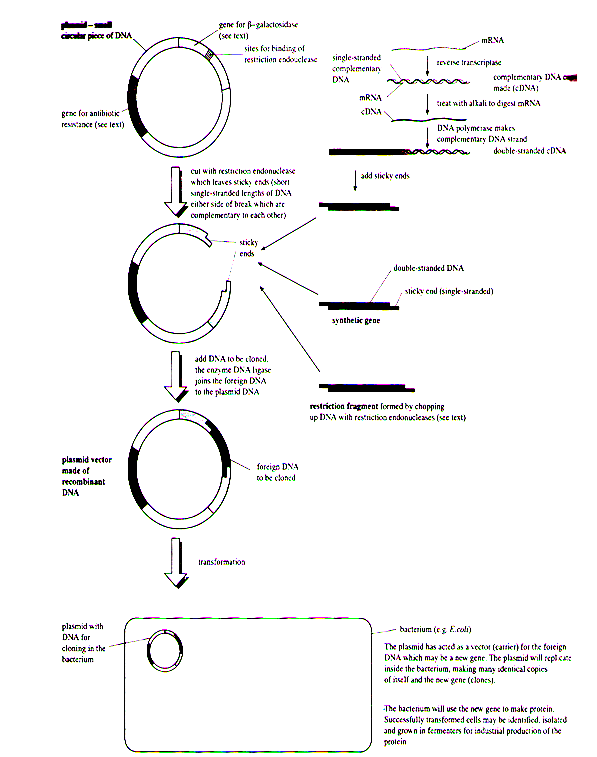
Genetic engineering. Summary of a procedure designed to clone a gene.
First Case Of Construction Of Artifical Recombinant DNA
Stanely Cohen and Herbert Boyer (1972) got the idea of linking a gene encoding for antibiotic resistance with a native plasmid of Salmonella typhimurium. Plasmids of bacteria are autonomously replicating circular extra chromosomal DNA bodies. They isolated antibiotic resistance gene by cutting a fragment of DNA from plasmid. This was done with the help of restriction enzymes popularly called as ‘molecular scissors’. This antibiotic resistant gene fragment was integrated with plasmid DNA.
These plasmid DNAs are responsible for transferring the piece of DNA attached to it to host cells, thus acted as vectors. DNA ligase enzyme helped in linking the fragment of DNA with vector. This newly formed circular DNA (formed in vitro) is called as recombinant DNA.
When this newly formed recombinant DNA was transferred to bacterium Escherichia coli, it was able to replicate in host cell by using DNA polymerase enzyme. As a result several copies of this recombinant DNA could be formed. This capability of antibiotic resistance gene in E. coli to form multiple copies is called as cloning of antibiotic resistance gene in E. coli.
For genetic modification of any organism, major requirements are:
- Identification of DNA with required genes.
- Introduction of desired identified genes into host.
- Maintenance of introduced DNA in the host
- Transfer of this DNA to its progeny
Tools Of Recombinant DNA Technology
Now we know from the foregoing discussion that genetic engineering or recombinant DNA technology can be accomplished only if we have the key tools, i.e., restriction enzymes, polymerase enzymes, ligases, vectors and the host organism. Let us try to understand some of these in detail.
Restriction Enzymes
Certain endonucleases, enzymes that cut DNA at specific DNA sequence within the molecule (as opposed to exonuclease, which digest from the ends of DNA molecules), are a key tool in recombinant DNA research. These enzymes were originally called restriction enzymes because their presence in a given bacterium restricted the growth of certain bacterial viruses called bacteriophages.
Restriction enzymes cut DNA of any source into short pieces in a sequence-specific manner, in contrast to most other enzymatic, chemical, or physical methods, which break DNA randomly. However, they are only present in cells that also have a companion enzyme that methylates the host DNA, rendering it an unsuitable substrate for digestion by the restriction enzyme. Thus, site-specific DNA methylases and restriction enzymes always exist in pairs in a bacterium. In the year 1963, the two enzymes responsible for restricting the growth of bacteriophage in Escherichia coli were isolated. One of these added methyl groups to DNA, while the other cut DNA. The later was called restriction endonuclease.
The first restriction endonuclease–Hind II, whose functioning depended on a specific DNA nucleotide sequence was isolated and characterized five years later. It was found that Hind II always cut DNA molecules at a particular point by recognizing a specific sequence of six base pairs. This specific base sequence is known as the recognition sequence for Hind II. Besides Hind II, today we know more than 900 restriction enzymes that have been isolated from over 230 strains of bacteria each of which recognize different recognition sequences.
Each restriction endonuclease functions by ‘inspecting’ the length of a DNA sequence. Once it finds its specific recognition sequence, it will bind to the DNA and cut each of the two strands of the double helix at specific points in their sugar - phosphate backbones. Each restriction endonuclease recognizes a specific palindromic nucleotide sequences in the DNA.
Palindromes are groups of letters that form the same word when read both forward and backward, e.g., “MALAYALAM”. As against a word-palindrome where the same word is read in both directions, the palindrome in DNA is a sequence of base pairs that reads same on the two strands when orientation of reading is kept the same. For example, the following sequences read the same on the two strands in 5' × 3' direction. This is also true if read in the 3' × 5' direction.
5' —— GAATTC —— 3'
3' —— CTTAAG —— 5'
Restriction enzymes cut the strand of DNA a little away from the centre of the palindrome sites, but between the same two bases on the opposite strands. This leaves single stranded portions at the ends.
Restriction enzymes are named after the bacterium from which they are isolated. For example, EcoRI is from Escherichia coli, and BamHI is from Bacillus amyloliquefaciens. The first three letters in the restriction enzyme name consist of the first letter of the genus (E) and the first two letters of the species (co). These may be followed by a strain designaton ® and a roman numeral (I) to indicate the order of discovery (e.g, EcoRI, EcoRII).
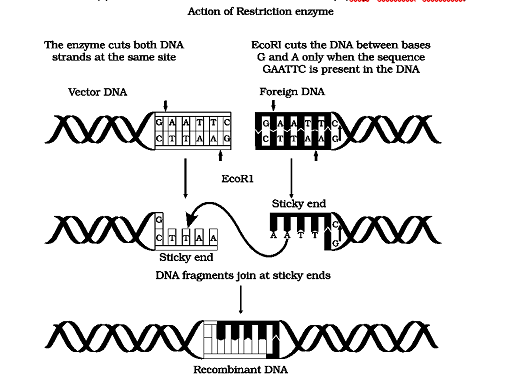
Steps in formation of recombinant DNA by action of restriction endonuclease enzyme – EcoRI
Each enzyme recognizes and cleaves a specific double-stranded DNA sequence that is 4-7 bp long. These DNA cuts result in blunt ends (Hpal) or overlapping (sticky) ends (BamHI), depending on the mechanism used by the enzyme. Sticky ends are particularly useful in constructing hybrid or chimeric DNA molecules. If the nucleotides are distributed randomly in a given DNA molecule, one can calculate how frequently a given enzyme will cut a length of DNA.
Reverse transcriptase enzyme:
It was discovered by Temin and Baltimore from retroviruses. Reverse transcriptase directs complementary DNA strand formation on the templates of m-RNA (RNA directed DNA synthesis or reverse of transcription process). It helps in in vitro synthesis of DNA or gene using specific m-RNA obtained from the cell. New gene can be obtained through this technique within a short duration using DNA polymerase enzyme and nucleotides of DNA molecules in triphosphate form.
DNA Polymerase Enzyme:
It is used for producing copies of DNA through PCR technique or replication of DNA under usual condition (DNA directed DNA synthesis). It was first discovered by Kornberg.
DNA ligase enzyme:
A type of enzyme used to seal the cut ends of DNA fragments. It is a type of molecular adhesive used during joining of desired gene with suitable vector DNA. DNA ligase was first discovered by Dr. Khorana from T4-bacteriophages and thus named as T4-ligase.
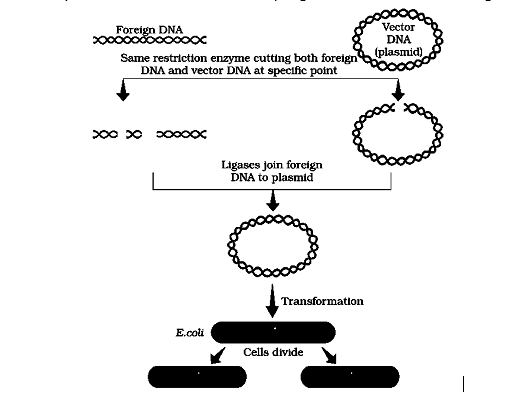
Diagrammatic representation of recombinant DNA technology We must have realised that normally, unless one cuts the vector and the source DNA with the same restriction enzyme, the recombinant vector molecule cannot be created.
Separation and isolation of DNA fragments
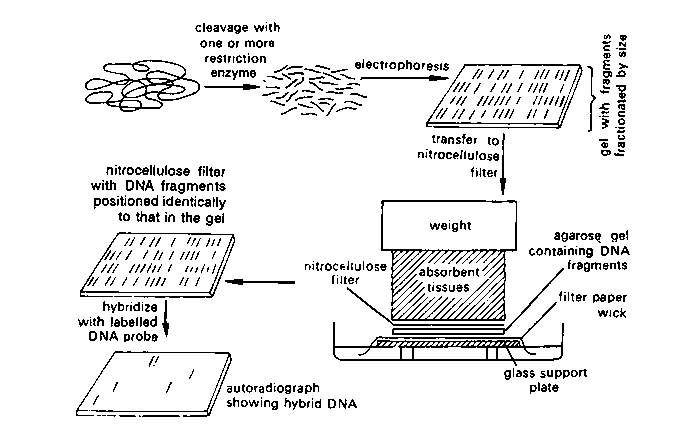
Gel electrophoresis separates macromolecules on the basis of their rate of movement through a gel under the influence of an electric field. In this method, mixtures of nucleic acids or proteins are placed in wells near one end of a thin slab of a polymeric gel. The gel is supported by glass plates and bathed in an aqueous solution. Electrodes are attached to both ends, and voltage is applied. Each macromolecule then migrates toward the electrode of opposite charge at a rate determined mostly by the molecule’s charge and size. Usually several different samples, each a mixture of molecules, are run simultaneously in multiple “lanes” on a slab gel, as shown here.
For nucleic acids, the rate of migration – how far a molecule travels while the current is on – is inversely proportional to molecular size. Nucleic acids carry negative charges (phosphate groups) proportionate to their lengths, but the gel impedes longer fragments more than it does shorter ones. The gel has been treated after electrophoresis with a DNA-binding dye that fluoresces pink in ultraviolet light. In each lane, the top of which is closest to the scientist, you can see a number of pink bands, which correspond to DNA molecules of different sizes. The larger molecules move more slowly through the gel and are located toward the bottom as in the photograph. The bands contain DNA restriction fragments. Twenty samples were run, each with a mixture of fragments from a DNA sample digested with a different restriction enzyme.
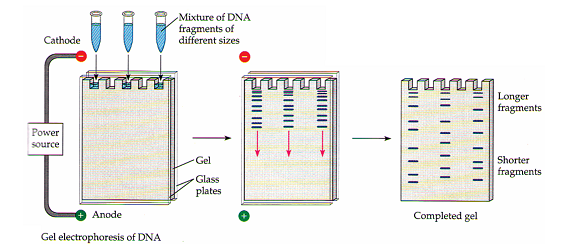
The separated DNA fragments can be visualized only after staining the DNA with a compound known as ethidium bromide followed by exposure to UV radiation (you cannot see pure DNA fragments in the visible light and without staining). You can see bright orange coloured bands of DNA in ethidium bromide stained gel exposed to UV light. The separated bands of DNA are cut out from the agarose gel and extracted from the gel piece. This step is known as elution. The DNA fragments purified in this way are used in constructing recombinant DNA by joining them with cloning vectors.
Cloning Vectors
We know that plasmids and bacteriophages have the ability to replicate within bacterial cells independent of the control of chromosomal DNA. Bacteriophages because of their high number per cell, have very high copy numbers of their genome within the bacterial cells. Some plasmids may have only one or two copies per cell whereas others may have 15-100 copies per cell. Their numbers can go even higher. If we are able to link an alien piece of DNA with bacteriophage or plasmid DNA, we can multiply its numbers equal to the copy number of the plasmid or bacteriophage. Vectors used at present, are engineered in such way that they help easy linking of foreign DNA and selection of recombinants from non-recombinants. The following are the features that are required to facilitate cloning into a vector.
(i) Origin of replication (ori): This is a sequence from where replication starts and any piece of DNA when linked to this sequence can be made to replicate within the host cells. This sequence is also responsible for controlling the copy number of the linked DNA. So, if one wants to recover many copies of the target DNA it should be cloned in a vector whose origin support high copy number.
(ii) Selectable marker: In addition to ‘ori’, the vector requires a selectable marker, which helps in identifying and eliminating non transformants and selectively permitting the growth of the transformants. Transformation is a procedure through which a piece of DNA is introduced in a host bacterium. Normally, the genes encoding resistance to antibiotics such as ampicillin, chloramphenicol, tetracycline or kanamycin, etc., are considered useful selectable markers for E. coli. The normal E. coli cells do not carry resistance against any of these antibiotics.
(iii) Cloning sites: In order to link the alien DNA, the vector needs to have very few, preferably single, recognition sites for the commonly used restriction enzymes. Presence of more than one recognition sites within the vector will generate several fragments, which will complicate the gene cloning.
Insertion of DNA into a functional region of the vector will interfere with the action of this region, and so care must be taken not to interrupt an essential function of the vector.
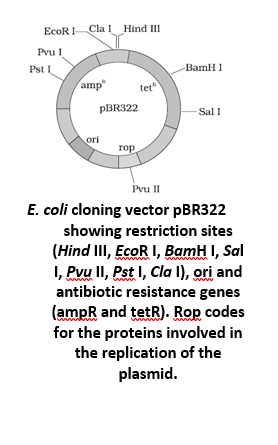
This concept can be exploited, however, to provide a selection technique. For example, the common plasmid vector pBR322 has both tetracycline (tet) and amplicillin (amp) resistance genes. A single Pstl site with the amp resistance gene is commonly used as the insertion site for a piece of foreign DNA. In addition to having sticky ends, the DNA inserted at this site disrupts the amp resistance gene and makes the bacterium carrying this plasmid amp sensitive. Thus, the parental plasmid, which provides resistance to both antibiotics, can be readily separated from the chimeric plasmid, which is resistant only to tetracycline.
In this case, one antibiotic resistance gene helps in selecting the transformants, whereas the other antibiotic resistance gene gets ‘inactivated due to insertion’ of alien DNA, and helps in selection of recombinants.
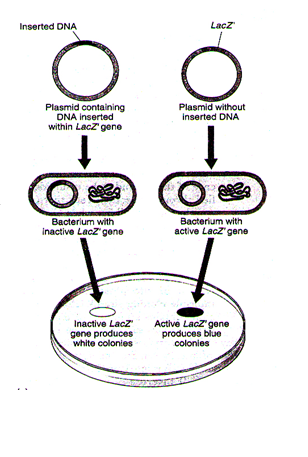
Insertion Inactivation Method
Selection of recombinants due to inactivation of antibiotics is a cumbersome procedure because it requires simultaneous plating on two plates having different antibiotics. Therefore, alternative selectable markers have been developed which differentiate recombinants from non-recombinants on the basis of their ability to produce colour in the presence of a chromogenic substrate. For example, in one method, the presence of inserted DNA results in inactivation of the LacZ gene. In this, a recombinant DNA is inserted within the coding sequence of an enzyme, a-galactosidase. This event can be detected by plating cells on medium containing X-gal, a substrate for the enzyme encoded by LacZ¢. Cells with an active version of the LacZ¢ gene will produce the enzyme and will be blue on this substrate, while cells that have an inactive LacZ¢ will lack the enzyme and will be white.
Phage Vectors
P hages are undoubtedly an excellent vector for carrying larger genes at a time (20-25 kb long genes or cluster of genes). Phage DNA is packed in proteinaceous head region and is linear and double stranded. It is nearly 50 kb long and can carry foreign DNA of approximately 25 kb length.
Cosmids
Cosmids have been constructed by combining certain features of plasmid and the ‘cos’ sites of phage lambda. (Collins and Bruning, 1978; Collins and Hohn, 1979). They have been constructed to add some of the advantages of phage vectors to the plasmid vectors’ the cos sites endeavour in vitro packaging system to the plasmid vector. The cosmid vectors, however, provide an efficient means of cloning large fragments of foreign DNA (32-48 kb of foreign DNA).
YAC Vectors
YACs or Yeast artificial chromosomes are being used as vectors to clone DNA fragments of more than 2500 Mb in size. They are being highly used in mapping larger genomes like Human Genome Project.
BAC Vectors
BACs or Bacterial artificial chromosomes are used as vectors which are based on natural extra-chromosomal plasmid of E. coli, the fertility or F-plasmid. This vector bears genes for replication and maintenance of F-factor, a selectable marker and cloning sites.
Shuttle Vectors
They are the plasmids capable of shuttling genes between two organisms. One of the organism is prokaryote like E. coli and other is a eukaryote like yeast. Such vectors should bear unique origins of replication for every cell type. They should have separate markers for transformed host cells harboring the vector.
Vectors for cloning genes in plants and animals
Ti plasmid of Agrobacterium tumefaciens (causing ‘crown gall’ disease; and Ri plasmid of A. rhizogenes (causing ‘hairy root’ disease) have been effectively used as vectors for gene transfer to plant cells. The Ti plasmid (and also Ri Plasmid) has a region called vir region (having six operons vir A, B, C, D, E, G) responsible for virulence towards host, and a T-DNA region, which is transferred to the host, along with the gene intended to be transferred.
Vectors based on Ti and Ri plasmids have been designed with the following properties:
- they do not cause the disease, since they are disarmed by removing genes causing the disease;
- they carry sites for insertion of foreign gene intended to be transferred and
- they carry selectable markers (genes which will help in selecting the transformed cells).
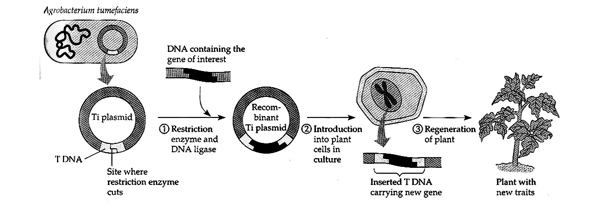
Using the Ti plasmid as a vector for genetic engineering in plants.
- The Ti plasmid is isolated from the bacterium Agrobacterium tumefaciens, and a fragment of foreign DNA is inserted into its T region by standard recombinant DNA techniques.
- When the recombinant plasmid is introduced into cultured plant cells, the T DNA integrates into the plant’s chromosomal DNA.
- As the plant cell divides, each of its descendants receives a copy of the T DNA and any foreign genes it carries. If an entire plant is regenerated, all its cells will carry – and may express – the new genes.
Competent Host (For Transformation with Recombinant DNA)
Since DNA is a hydrophilic molecule, it cannot pass through cell membranes. In order to force bacteria to take up the plasmid, the bacterial cells must first be made ‘competent’ to take up DNA. This is done by treating them with a specific concentration of a divalent cation, such as calcium, which increases the efficiency with which DNA enters the bacterium through pores in its cell wall. Recombinant DNA can then be forced into such cells by incubating the cells with recombinant DNA on ice, followed by placing them briefly at 42°C (heat shock), and then putting them back on ice. This enables the bacteria to take up the recombinant DNA. This is not the only way to introduce alien DNA into host cells.
Other methods to introduce alien DNA into host cell are microinjection, electroporation, gene gun method and Ti-plasmid-based transfer.
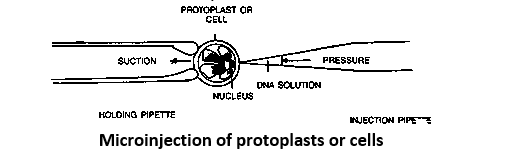
- Microinjection. DNA can also be stably introduced into tissue culture cells by its direct microinjection into the nuclei of the cells, using a glass micropipette that has been drawn out to an extremely thin diameter (from 0.1 to 0.5 microns). It is found that injected DNA will integrate at random into the nuclear DNA, and if an injected gene is attached to a suitable promoter it might be expressed. The advantage of this procedure is that in principle any piece of DNA can be introduced into any cell.
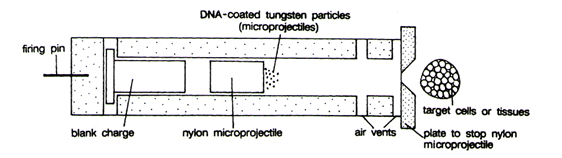
- Shot-gun/gene gun method of DNA introduction – In recent years, it has been shown that DNA delivery to plant cells is also possible, when heavy metallic pellets (tungsten or gold) coated with the DNA of interest are accelerated to a very high initial velocity (1,400 ft/sec). These microprojectiles, normally 1-3 mm in diameter, are carried by a ‘macroprojectile’ or the ‘bullet’ and accelerated into living plant cells (target cells can be pollen, cultured cells, cells in differentiated tissues and meristems) so that they can penetrate cells walls of intact tissue.
- The tumor inducing (Ti) plasmid of Agrobacterium tumefaciens has now been modified into a cloning vector which is no more pathogenic to the plants but is still able to use the mechanisms to deliver genes of our interest into a variety of plants. Similarly, retroviruses have also been disarmed and are now used to deliver desirable genes into animal cells. So, once a gene or a DNA fragment has been ligated into a suitable vector it is transferred into a bacterial, plant or animal host (where it multiplies).
Processes Of Recombinant DNA Technology
Recombinant DNA technology involves several steps in specific sequence such as isolation of DNA, fragmentation of DNA by restriction endonucleases, isolation of a desired DNA fragment, ligation of the DNA fragment into a vector, transferring the recombinant DNA into the host, culturing the host cells in a medium at large scale and extraction of the desired product. Let us examine each of these steps in some details.

Isolation of the Genetic Material (DNA)
Nucleic acid is the genetic material of all organisms without exception. In majority of organisms this is deoxyribonucleic acid or DNA. In order to cut the DNA with restriction enzymes, it needs to be in pure form, free from other macro-molecules. Since the DNA is enclosed within the membranes, we have to break the cell open to release DNA along with other macromolecules such as RNA, proteins, polysaccharides and also lipids. This can be achieved by treating the bacterial cells/plant or animal tissue with enzymes such as lysozyme (bacteria), cellulase (plant cells), chitinase (fungus).
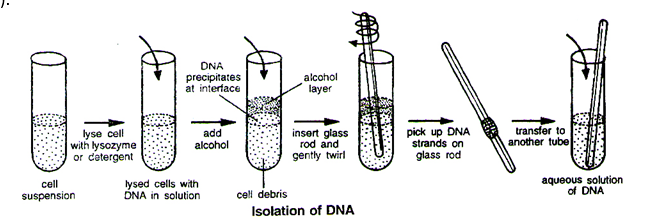
We know that genes are located on long molecules of DNA interwined with proteins such as histones. The RNA can be removed by treatment with ribonuclease whereas proteins can be removed by treatment with protease. Other molecules can be removed by appropriate treatments and purified DNA ultimately precipitates out after the addition of chilled ethanol. This can be seen as collection of fine threads in the suspension.
Cutting of DNA at Specific Locations
Restriction enzyme digestions are performed by incubating purified DNA molecules with the restriction enzyme, at the optimal conditions for that specific enzyme. Agarose gel electrophoresis is employed to check the progression of a restriction enzyme digestion. DNA is a negatively charged molecule, hence it moves towards the positive electrode (anode). The process is repeated with the vector DNA also. The joining of DNA involves several processes. After cutting the source DNA as well as the vector DNA with a specific restriction enzyme, the cut out ‘gene of interest’ from the source DNA and the cut vector with space are mixed and ligase is added. This results in the preparation of recombinant DNA.
Amplification of Gene of Interest using PCR
Once a particular gene is identified within the library of DNA fragments, the final requirements is to make multiple copies of it. One way to do this is to insert the identified fragment into a bacterium; after repeated cell divisions, millions of cells will contain copies of the fragment.
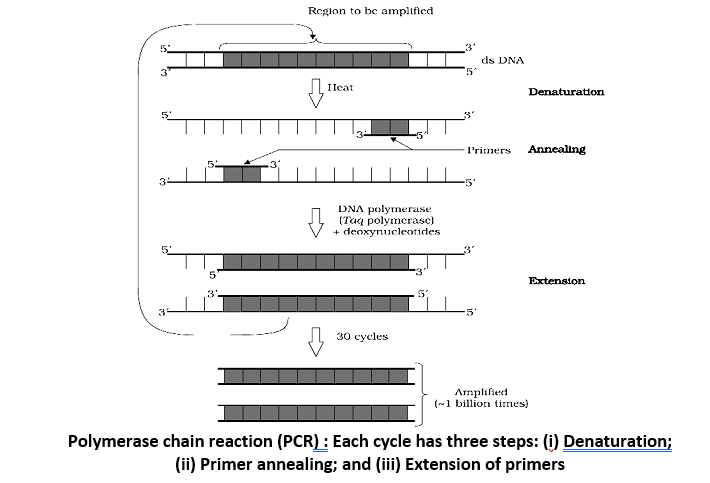
A far more direct approach, however, is to use DNA polymerase to copy the gene sequene of interest through the polymerase chain reaction. Kary Mullis developed PCR in 1983. In 1993 his discovery won him the Noble Prize in chemistry. PCR can amplify specific sequences or add sequences (such as endonuclease recognition sequences) as primers to cloned DNA. There are three steps in PCR:
- Step 1: Denaturation. First, an excess of primer (typically, a synthetic sequence of 20 to 30 nucleotides) is mixed with the DNA fragment to be amplified. This mixture of primer and fragment is heated to about 98°C. At this temperature, the double-stranded DNA fragment dissociates into single strands.
- Step 2: Annealing of Primers. Next, the solution is allowed to cool to about 60°C. As it cools, the single strands of DNA re-associate into double strands. However, because of the large excess of primer, each strand of the fragment base-pairs with the complementary primer flanking the region to be amplified, leaving the rest of the fragment single-stranded.
- Step 3: Primer Extension - Now a very heat-stable type of DNA polymerase, called Taq polymerase (after the thermophilic bacterium Thermus aquaticus, from which Taq is extracted) is added, along with a supply of all four nucleotides. Using the primer, the polymerase copies the rest of the fragment as if it were replicating DNA. When it is done, the primer has been lengthened into a complementary copy of the entire single-stranded fragment. Because both DNA strands are replicated, there are now two copies of the original fragments.
Insertion of Recombinant DNA into the Host Cell/Organism
There are several methods of introducing the ligated DNA into recipient cells. Recipient cells after making them ‘competent’ to receive, take up DNA present in its surrounding. So, if a recombinant DNA bearing gene for resistance to an antibiotic (e.g., ampicillin) is transferred into E. coli cells, the host cells become transformed into ampicillin-resistant cells. If we spread the transformed cells on agar plates containing ampicillin, only transformants will grow, untransformed recipient cells will die. Since, due to ampicillin resistance gene, one is able to select a transformed cell in the presence of ampicillin. The ampicillin resistance gene in this case is called a selectable marker.
Obtaining the Foreign Gene Product
- Bioreactor. A chamber within which the activity of a biocatalyst can be optimally controlled, often for industrial production. They include fermenters such as the chemostat. In batch fermentation, the nutrients and microorganism are put into a closed reactor, nothing is added while fermentation occurs and nothing except waste gases leaves the chamber, and most environmental conditions except temperature are normally allowed to vary unchecked.
The product is separated from the micro-organisms when nutrients have been utilized; the exponential growth phase usually lasts only a short time, and secondary metabolities such as antibiotics (e.g. penicillin) are produced only towards the end of the exponential growth phase, risks of contamination being reduced compared with continuous culturing methods. In the fed batch method, the first stage involves the above batch method but then controlled feeding is applied in order to reduce growth rate compactly with the oxygen supply, resulting in good quality protein production.
Continuous fermentation is less often employed than batch fermentation. Here open reactors (chemostats) are used and nutrients added continuously at a rate which balances their removal rate. Microorganisms are thus kept in their exponential growth phase, for which monitoring and constancy of environmental variables (pH, temperature, O2, substrate, product and waste levels) are essential.
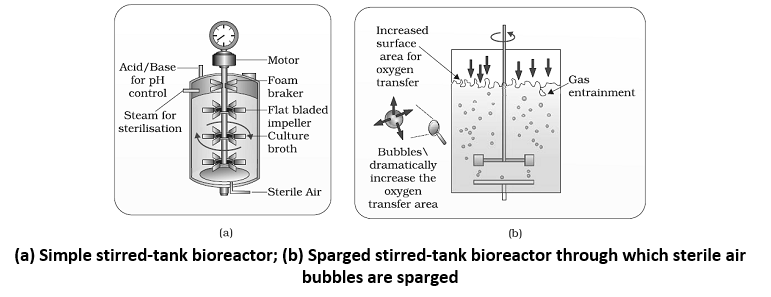
- To produce in large quantities, the development of bioreactors, where large volumes (100 – 1000 litres) of culture can be processed, was required.
- A bioreactor provides the optimal conditions for achieving the desired product by providing optimum growth conditions (temperature, pH, substrate, salts, vitamin, oxygen)
- The most commonly used bioreactors are of stirring type. A stirred-tank reactor is usually cylindrical or with a curved base to facilitate the mixing of the reactor contents. The stirrer facilitate the mixing of the reactor contents. The stirrer facilitates even mixing and oxygen availability throughout the bioreactor. Alternatively air can be bubbled through the reactor. The bioreactor has an agitator system, an oxygen delivery system and a foam control system, a temperature control system, pH control system and sampling ports so that small volumes of the culture can be withdrawn periodically.
Downstream Processing
- After completion of the biosynthetic stage, the product has to be subjected through a series of processes before it is ready for marketing as a finished product.
- The processes include separation and purification, which are collectively referred to as downstream processing.
- It is an essential step in the manufacture of pharmaceuticals such as antibiotics, hormones (e.g., insulin and human growth hormone), antibodies, vaccines, etc.
- The product has to be formulated with suitable preservatives
- Strict quality control testing for each product is also required.
- The downstream processing and quality control testing vary from product to product.
Biotechnology Applications
Biotechnology, essentially deals with industrial scale production of biopharmaceuticals and biologicals, using genetically modified microbes, fungi, plants and animals. The applications of biotechnology include therapeutics, diagnostics, genetically modified crops for agriculture, processed food, bioremediation, waste treatment, and energy production. Three critical research areas of biotechnology are:
(i) Providing the best catalyst in the form of improved organism usually a microbe or pure enzyme.
(ii) Creating optimal conditions through engineering for a catalyst to act, and
(iii) Downstream processing technologies to purify the protein/organic compound.
Let us now learn how human beings have used biotechnology to improve the quality of human life, especially in the field of food production and health.
Applications in Agriculture
- Agricultural biotechnology is the area of biotechnology involving applications to agriculture.
- Agricultural biotechnology has been practiced for a long time, as people have sought to improve agriculturally important organisms by selection and breeding.
- An example of traditional agricultural biotechnology is the development of disease-resistant wheat varieties by cross-breeding different wheat types until the desired disease resistance was present in a resulting new variety.
- Food production can be increased by:
- i) agro-chemical based agriculture
- ii) organic agriculture
iii) genetically engineered crop-based agriculture
- Green revolution resulted in great increase in production of food grains (as rice and wheat) due to the introduction of high-yielding varieties, use of pesticides to better management techniques and use of agrochemical (fertilizers and pesticides).
- For farmers in the developing world, agrochemicals are often too expensive. Further increases in yield with existing varieties are not possible using conventional breeding.
- Microbes, plants and animals whose genes have been altered by manipulation are called “Genetically Modified Organisms (GMO)”.
- USES OF GM PLANTS - GM plants have been useful in many ways.
Genetic modification has:
- i) made crops more tolerant to abiotic stresses (cold, drought, salt, heat, etc.)
- ii) reduced reliance on chemical pesticides (pest-resistant crops)
iii) helped to reduce post harvest losses
- iv) increased efficiency of mineral usage by plants (this prevents early exhaustion of fertility of soil).
- v) enhanced nutritional value of food, e.g., Vitamin ‘A’ enriched rice
- GM has been used to create tailor-made plants to supply alternative resources to industries, in the form of starches, fuels and pharmaceuticals.
- The production of pest resistant plants, could decrease the amount of pesticide used.
- Bt toxin is produced by a bacterium called Bacillus thuringiensis (Bt for short).
Bt toxin is produced by cloning the gene responsible for it from the bacteria and expressing the same in plants to provide resistance to insects, without the need for insecticides; this in effect created a bio-pesticide. e.g., Bt cotton, Bt corn, rice, tomato, potato and soyabean.
- Foods derived from transgenic plants have been called “GMO foods,” “GMPs” (genetically modified products), and “biotech foods.”
- While some refer to foods developed from genetic engineering technology as “biotechnology-enchanced foods,” others call them “Franken foods.”
Bt Cotton
Insect resistant plants can also be developed by RDT. Progress in engineering insect resistance features in transgenic plants has been made through the expression of insect toxin gene of Bacillus thuringiensis in plants. Most strains of this toxin gene are toxic to lepidopteran insect larvae. The toxicity is due to toxin gene named as Bt - 2.
Some strains of Bacillus thuringiensis produce proteins that kill certain insects such as
- Lepidopterans – e.g. Tobacco budworm, armyworm
- Coleopterans (beetles) and
- Dipterans – e.g. Flies, mosquitoes
- thuringiensis forms certain protein crystals during a particular phase of their growth. These crystals contain a toxic insecticidal protein.
The Bt toxin protein does not cause any damage to the bacterium as it exist as inactive protoxins within the Bacillus, but once an insect ingest the inactive toxin, it is converted into an active form of toxin due to the alkaline pH of insect gut which solubilise the crystals. The activated toxin binds to the surface of mid gut epithelial cells and create pores that cause cell swelling and lysis and eventually cause death of the insect.
Bt Toxin –
Specific Bt toxin genes were isolated from Bacillus thuringiensis and incorporated into the several crop plants such as cotton. The choice of genes depends upon the crop and the targeted pest, as most Bt toxins are insect-group specific. The toxin is coded by a gene named as cry. There are a number of them, for example, the proteins encoded by the genes cryIAc and cryIIAb control the cotton bollworms, whereas that of cryIAb controls corn borer. The protein formed by gene cry II Ab controls the Colorado potato beetle and Cry III Bb controls corn root worm.
Cry genes & their targets
| Cry Genes | Target Insects |
| CryIA – CryIE | Lepidoptera |
| CryIIA | Lepidoptera & Diptera |
| CryIIB, CryIVA – CryIVD | Diptera |
| CryIIIA – CryIIIC | Coleoptera |
| CryV, CryVI | Nematodes |
Bt toxin gene has been cloned from the bacteria and expressed in plants to provide resistance from insects without the requirement of insecticides. It has created a type of biopesticide e.g. Bt cotton, Bt corn, rice, tomato, potato and soyabean etc.
- thuringiensis produces some types of protein crystals in specific stage of their growth and development. The gene encoding cry protein i.e., cry gene has been isolated and transferred into several crops. This is case where transgene product directly produces the phenotype of interest.

Cotton boll: (a) destroyed by bollworms; (b) a fully mature cotton boll
- Transgenic plants: Resistance against bacterial and fungal pathogens
Several examples are now available, where genes imparting resistance against bacterial and fungal pathogens have been successfully transferred to tomato (against Pseudomonas, Alternaria and Rhizoctonia) and potato (against Phytophthora).
- Transgenic tomato plants for hard skin and improved flavor:
In tomato using antisense RNA technology, transgenic plants have been produced which are either ‘bruise resistant’ (suitable for transport and storage) or exhibit ‘delayed ripening’ giving more time for ripening on the plant, thus permitting more time for sugar accumulation and also giving higher shelf life. These are given the name ‘Flavr Savr’ tomato.
Pest Resistant Plants (RNA interference Method)
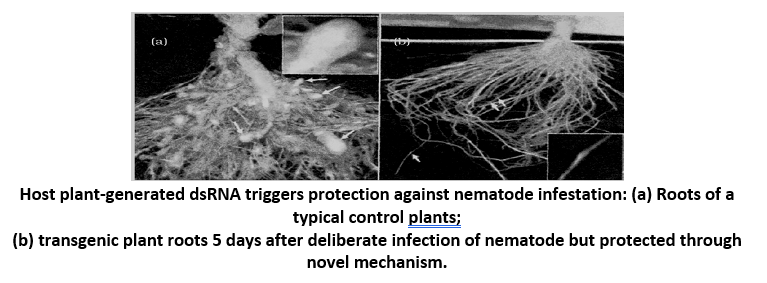
Several nematodes parasitizes a wide variety of plants and animals including human beings. A root-knot nematode, Meloidogyne incognita is an important pest of vegetables, cereals, ornamentals, pasture, trees and shrubs, sugarcane, tobacco, cotton, potatoes, etc. It attacks almost all the cultivated plants and can cause high losses. A technique to prevent this infestation is based on the process of RNA interference (RNAi).
RNA interference (RNAi) is a process which involves the introduction of double-stranded RNA into a cell inhibiting the expression of genes. RNAi takes place in all eukaryotic organisms as a method of cellular defense.
Process of RNA interference - This method involves silencing of a specific mRNA due to a complementary ds RNA molecule that binds to and prevents translation of the mRNA (silencing).The complementary RNA could be obtained from viruses having RNA genomes or mobile genetic elements (transposons) that replicate via an RNA intermediate.
Using Agrobacterium vectors, nematode-specific genes were introduced into the host plant. The introduction of DNA was such that it produced both sense and anti-sense RNA in the host cells. These two RNA’s being complementary to each other formed a double stranded (ds RNA) that initiated RNAi and thus, silenced the specific mRNA of the nematode. The consequence was that the parasite could not survive in a transgenic host expressing specific interfering RNA. The transgenic plant therefore got itself protected from the parasite.
The Noble Prize in Physiology or Medicine 2006 was awarded jointly to Andrew Z. Fire and Craig C. Mello for their discovery of RNA interference-gene silencing by double-stranded RNA.
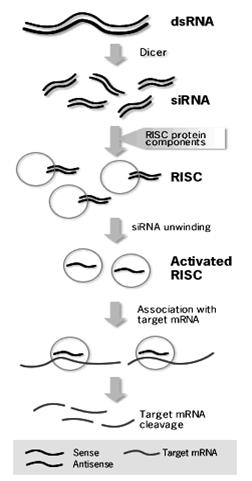
The Mechanism of RNA Interference (RNAi)
Long double-stranded RNAs (dsRNAs; typically >200 nt) can be used to silence the expression of target genes in a variety of organisms and cell types (e.g., worms, fruit flies, and plants).
Upon introduction, the long ds RNAs enter a cellular pathway that is commonly referred to as the RNA interference (RNAi) pathway.
First, the ds RNAs get processed into 20-25 nucleotide (nt) small interfering RNAs (siRNAs) by an RNase III-like enzyme called Dicer (initiation step).
Then, the siRNAs assemble into endoribonuclease - containing complexes known as RNA-induced silencing complexes (RISCs), unwinding in the process.
The siRNA strands subsequently guide the RISCs to complementary RNA molecules, where they cleave and destroy the cognate RNA (effecter step). Cleavage of cognate RNA takes place near the middle of the region bound by the siRNA strand. In mammalian cells, introduction of long dsRNA (>30 nt) initiates a potent antiviral response, exemplified by nonspecific inhibition of protein synthesis and RNA degradation. The mammalian antiviral response can be bypassed, however, by the introduction or expression of siRNAs
Biotechnological Applications In Medicine
The recombinant DNA technological processes have made immense impact in the area of healthcare by enabling mass production of safe and more effective therapeutic drugs. Further, the recombinant therapeutics do not induce unwanted immunological responses as is common in case of similar products isolated from non-human sources. At present, about 30 recombinant therapeutics have been approved for human-use the world over. In India, 12 of these are presently being marketed.
Genetically Engineered Insulin
Insulin is a protein hormone made in the pancreas which plays a vital role in the regulation of blood sugar levels. Its deficiency is one of the causes of the disease diabetes mellitus (sugar diabetes) where blood sugar levels become raised with harmful consequences.
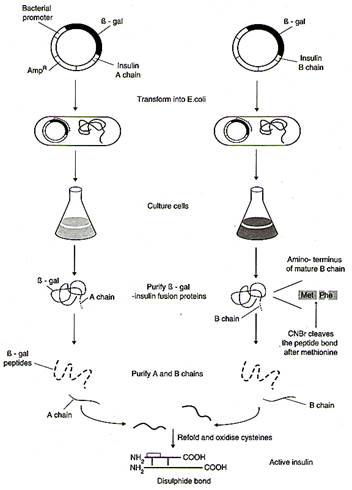
Daily injections of insulin isolated from the pancreas of slaughtered pigs and cattle became the standard treatment. However, due to minor differences in the amino acid composition of insulin from species different to ourselves, and to traces of impurities, some patients were allergic to animal insulin and showed damaging side effects as a result of the injections. The ideal solution became possible with the introduction of genetic engineering. The gene for human insulin is inserted into a bacterium, and the bacterium is grown in a fermenter to make large quantities of the protein. An outline of the procedure currently used for human insulin production is shown in figure.
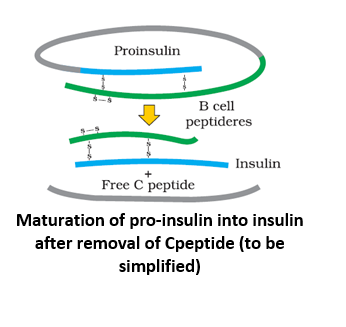
Insulin consists of 51 amino acids forming two short polypeptide chains - chain A having 21 amino acids and chain B with 30 amino acids. The two chains are linked by disulfide bond. In animals, including humans, insulin occurs as proinsulin. It is made of chain A, chain B and chain C (30 amino acids). As the insulin matures, chain C is removed.
The genetic engineering of insulin begins with identification and separation of DNA sequences coding for chain A and chain B. This was found to be present at the top of the short arm of the eleventh chromosome. It contains 153 nucleotides-63 nucleotides for chain A and 90 nucleotides for chain B.
|
The expression of genes for human insulin A and B polypeptide chains in E. coli. The two genes are expressed in separate bacterial systems. The A and B chains are then refolded and joined covalently to form the mature protein, insulin. |
These sequences were introduced into plasmid (pBR322) of Escherichia coli - common human colon bacterium. It is said to be the factory used in genetic engineering of insulin. In E. coli, -galactosidase controls the transcription of these genes, therefore, insulin gene needs to be tied to this enzyme. The protein formed by E. coli consists partly of -galactosidase joined to either A or B chain of insulin.
These are then extracted from -galactosidase fragment and purified. The two chains are mixed and reconnected in a reaction that forms disulfide bridges resulting in pure humulin-the synthetic human insulin.
Development Of Humulin
The original technique was developed by Eli Lilly and Company and in 1982 human insulin, marketed as ‘humulin’, became the first genetically engineered pharmaceutical product to be approved for use.
Gene Therapy
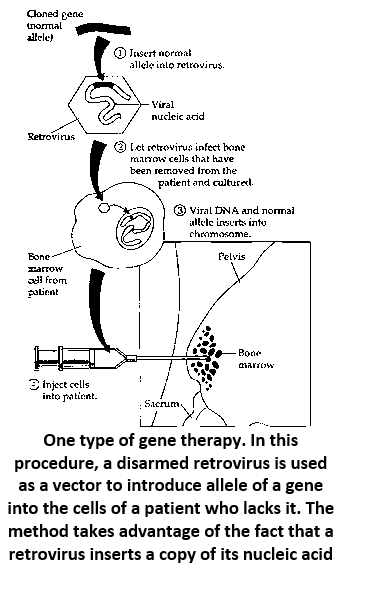
Genetic engineering has the potential to actually correct some genetic disorders in individuals. For any genetic disorder traceable to a single defective allele, it should theoretically be possible to replace or supplement the defective allele with a functional, normal allele using recombinant DNA techniques. The new allele could be inserted into the somatic cells of a child or adult, or into germ (gamete-producing) cells or embryonic cells.
Gene therapy is especially suited to the treatment of single-enzyme deficiency diseases, where an introduced normal allele would provide the missing enzyme. In this case, normal alleles must be introduced into a patient's own somatic cells that actively reproduce in the body (so that the normal allele will be replicated in the individual) and whose normal protein products can correct the disorder. It may not matter precisely how many engineered cells are introduced into the body as long as a minimum amount of the product is produced.
- The use of bioengineered cells or other biotechnology techniques to treat human genetic disorders is known as gene therapy.
- Gene therapy is the transfer of normal genes into body cells to correct a genetic defect.
- ADA deficiency is a genetic disease and individuals with this disease inherit defective ADA genes and are unable to produce the enzyme adenosine deaminase in their cells.
- ADA deficiency leads to a severe combined immunodeficiency (ADA-SCID).
- The disease is caused by a mutation of ADA in a gene located on the long arm of chromosome 20.
- The gene codes for the enzyme adenosine deaminase (ADA).
- Without this enzyme, the body is unable to break down a toxic substance called deoxyadenosine.
- The enzyme adenosine deaminase is essential to the proper functioning of the immune system.
- The toxin builds up and destroys infection-fighting immune cells called T and B lymphocytes.
- Patients of ADA are more susceptible to all kinds of infection, particularly those of the skin, respiratory system and gastro-intestinal tract.
- Most babies who are born with the disorder die within a few months.
Molecular Diagnosis
We know that for effective treatment of a disease, early diagnosis and understanding its pathophysiology is very important. Using conventional methods of diagnosis (serum and urine analysis, etc.) early detection is not possible. Recombinant DNA technology, Polymerase Chain Reaction (PCR) and Enzyme Linked Immuno - sorbent Assay (ELISA) are some of the techniques that serve the purpose of early diagnosis.
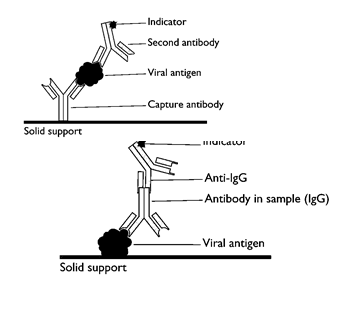
ELISA (Principle and General procedure) ELISA is based on the ability of low molecular weight antibodies to couple with enzymes, to produce enzymatically active immunological conjugates. This allows the detection of immune reaction with histochemical staining techniques because the antibody component is involved in immune reaction and the conjugated enzyme can be used for staining reaction using appropriate substrate. It can be used to detect antigen as well as antibodies in the serum of patient.
Probe Method
Methods for detecting a gene directly all depend on base pairing between the gene and a complementary sequence on another nucleic acid molecule, a process called hybridization. The complementary molecule, which can be either RNA or DNA, is called a probe. When at least part of the nucleotide sequence of the gene is known, or can be deduced from knowledge of the amino acid sequence of the gene's protein product, short probes complementary to it can be chemically synthesized. The probe, which will hydrogen-bond specifically to the desired gene, can be traced by labeling it with a radioactive isotope or a fluorescent tag.
The clone having the mutated gene will hence not appear on the photographic film, because the probe will not have complementarity with the mutated gene. ELISA is based on the principle of antigen-antibody interaction. Infection by pathogen can be detected by the presence of antigens (proteins, glycoproteins, etc.) or by detecting the antibodies synthesized against the pathogen.
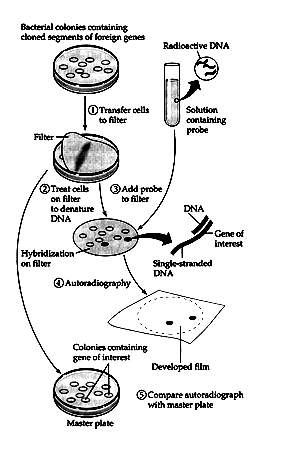
Hybridization
We briefly examined nucleic acid hybridization in the context of probes used to identify specific clones. The technique can be applied to a wider variety of problems. Consider an example: a researcher has just identified and cloned a gene that plays an important regulatory role during mitosis in yeast cells. Do other organisms - for example, humans, or mice, or frogs-also contain this gene? Hybridization can be used to address this question. The basis of the technique is to allow a labeled probe, a specific piece of DNA complementary to the gene of interest, to bind to the DNA from various cells being tested. If such complementary sequences are present, the probe will base-pair to them. Cells carrying the gene can then be identified because the probe has been labeled.
With hybridization, researchers can learn more about the complementary sequence than simply whether it is present or not. The Methods Box on p. 381 describes in more detail a particular kind of hybridization method called Southern hybridization, or Southern blotting. This technique enables a researcher to determine not only whether a particular sequence is present within a sample of DNA, but how many such sequences are there, and the size of the restriction fragments that contain these sequences.
Messenger RNA can also be subjected to hybridization analysis, in an analogous process known as Northern blotting. Typically, the goal is to determine whether a particular gene is made into mRNA, how much of that mRNA is present, and whether the abundance of that specific mRNA changes at different stages of development or in response to certain regulatory signals. Such Northern hybridization has become the mainstay of research focusing on the control of gene expression.
Application Of Molecular Diagnosis
- Very low concentration of bacteria or virus (pathogen) at the time, when symptoms of disease are not visible can be detected by amplification of their nucleic acid by Polymerase Chain Reaction (PCR).
- PCR technique is being widely used to detect HIV in suspected AIDS patients.
- PCR technique is used to detect mutations in suspected cancer patients.
- Cloned genes are used as probes to detect the presence of its complementary DNA strands.
- ELISA technique is widely used to detect viruses, fungi, bacteria, mycoplasma like organisms.
- ELISA test is also used to diagnose diseases like tuberculosis, AIDS etc.
Transgenic Animals
Describing an organism whose normal genome has been altered by introduction of a gene (transgene) by a manipulative technique (microinjection of DNA into egg or whole organism; use of plasmid of virus based DNA vector), often involving introduction of DNA from a different species. The foreign DNA may then integrate into the host genome. Such organisms are potentially valuable in plant and animal husbandry. The technique is yielding results in the genetics of animal development.
The majority of transgenic animals produced so far are mice, the animal that pioneered the technology. The first successful transgenic animal was a mouse. A few years later, it was followed by rabbits, pigs, sheep and cattle.
The transgenesis involves the transfer of desired isolated gene or gene fragments or individual chromosome or chromosomal fragments, or isolated nuclei from one organism to another organism.
Significance Of Transgenesis
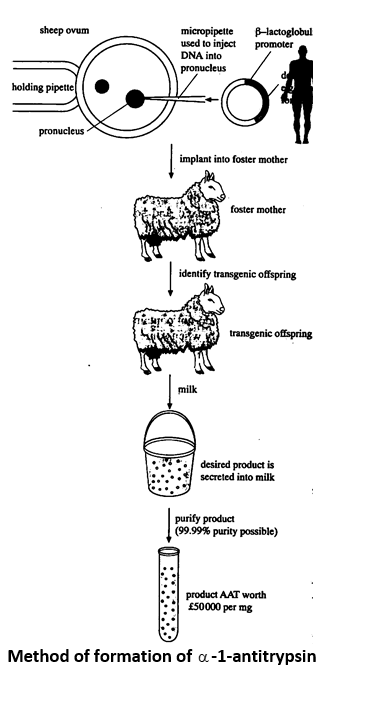
(i) Role of transgenesis in molecular farming. Molecular farming involves the extraction of useful proteins and drugs from the milk, food and urine of transgenic animals which may be used as bioreactors e.g.
- Transgenic sheep having human antitrypsin gene ( AT) was produced with the help of ovine -lactoglobulin gene promotor. Transgenic ewes produce AT protein in their milk and can be used against the
- Other examples of proteins produced in this way include factor IX, a blood-clotting protein whose absence causes one type of haemophilia. Another is tPA (tissue plasminogen activator) which is used to dissolve blood clots in patients suffering from heart disease.
(ii) Increased production of biological products
- In 1997, first transgenic cow, named Rosie, with human alpha-lactalbumin gene was produced. The milk of transgenic cow contained about 2.4 grams of human protein per litre of milk and was found to be more nutritionally balanced product for human babies than that of natural cow milk. Such human milk proteins can be extracted and used pharmaceutically.
- In 1985, the transgenic fishes of many species like common carp, catfish, goldfish, salmon, Tilapia, rainbow trout and zebra fish have been produced by microinjection of genes coding for rat or human growth hormone (rGH or hGH). It was found that transgenic fish with hGH gene was found to be twice in size than the non-transgenic fish.
(iii) Study of diseases and gene therapy
- These act as models for human diseases like cancer, cystic fibrosis, rheumatoid arthritis, Alzheimer's disease, etc, and their possible new methods of their treatment.
- Transfection of cultured mammalian cells have been used extensively for detecting the cancer genes (oncogenes) and their gene therapy. In these retroviruses, adeno-associated virus (AAVs) and naked DNA have been used as vectors and gene therapy.
(iv) Vaccine safety testing. Transgenic mice are first to be used as laboratory animals to test the efficacy of a newly discovered vaccine before it is used on human beings e.g. polio vaccine. If such vaccines are found satisfactory and reliable on mice, then these are tested on the monkeys much closely related to man.
(v) Chemical safety testing. For this transgenic animals with foreign genes are produced so that the transgenic animals become more sensitive to the toxic chemicals of them, the non-transgenic animals. Then these animals are exposed to toxic chemicals and their effects are observed. The time required to obtain the results is less.
ETHICAL ISSUES
Ethical issues includes a set of standards by which a community regulates its behaviour and decides as to which activity is legitimate and which is not. Therefore bioethics may be viewed as a set of standards that may be issued to regulate our activities in relation to the biological world.
Therefore, the Indian Government has set up organizations such as GEAC (Genetic Engineering Approval Committee), which will make decisions regarding the validity of GM research and the safety of introducing GM organisms for public services.
- Biopatent: A patent is a right granted by a government to an inventor to prevent others from commercial use of his invention. A patent is granted for:
Most of the industrialized nations are rich financially but poor in biodiversity and traditional knowledge. In contrast the developing and the underdeveloped world is rich in biodiversity and traditional knowledge related to bio-resources. Traditional knowledge related to bio-resources can be exploited to develop modern applications and can also be used to save time, effort and expenditure during their commercialization. There has been growing realization of the injustice, inadequate compensation and benefit sharing between developed and developing countries. Therefore, some nations are developing laws to prevent such unauthorized exploitation of their bio-resources and traditional knowledge.
The Indian Parliament has recently cleared the second amendment of the Indian Patents Bill, which takes such issues into consideration, including patent terms emergency provisions and research and development initiative.
- (A) An invention (including product)
- (B) An improvement in an earlier invention
- (C) The process of generating products
- (D) A concept or design
These products are called bio-patents because they are granted for biological entities.
Biopatents are awarded for the following:
- (i) strains of microorganisms
- (ii) cell lines
- (iii) genetically modified strains of plants and animals,
- (iv) DNA sequences
- (v) the proteins encoded by DNA sequences
- (vi) various biotechnological procedures
- (vii) production processes
- (viii) products and
- (ix) product applications
For example, one patent covers 'all transgenic plants of Brassica family'. Such board patents are considered morally unacceptable and fundamentally inequitable.
- Biopiracy: Many organizations and multinational companies exploit or patent biological resources or bio-resources of other nations without proper authorization from the countries concerned and this is known as biopiracy.
The industrialized nations are rich in technology and financial resources but poor in biodiversity and traditional knowledge related to the utilization of the bio-resources. In contrast, developing nations are poor in technology and financial resources, but are rich in biodiversity and traditional knowledge related to bioresources. There are an estimated 200,000 varieties of rice in India alone. The diversity of rice in India is one of the richest in the world. Basmati rice is distinct for its unique aroma and flavour and 27 documented varieties of Basmati are grown in India.
In 1997, an American company got patent rights on Basmati rice through the US Patent and Trademark Office. This allowed the company to sell a ‘new’ variety of Basmati, in the US and abroad. This ‘new’ variety of Basmati had actually been derived from Indian farmer’s varieties. Indian Basmati was crossed with semi-dwarf varieties and claimed as an invention or a novelty. The patent extends to functional equivalents, implying that other people selling Basmati rice could be restricted by the patent. Several attempts have also been made to patent uses, products and processes based on Indian traditional herbal medicines, e.g., turmeric neem. If we are not vigilant and we do not immediately counter these patent applications, other countries/individuals may encash on our rich legacy and we may not be able to do anything about it.
There is growing public anger that certain companies are being granted patents for products and technologies that make use of the genetic materials, plants and other biological resources that have long been identified, developed and used by farmers and indigenous people of a specific region/country. Rice is an important food grain, the presence of which goes back thousands of years in Asia’s agricultural history. There is reference to Basmati in ancient texts, folklore and poetry, as it has been grown for centuries