BIOTECHNOLOGY: APPLICATION
Biotechnology, essentially deals with industrial scale production of biopharmaceuticals and biologicals, using genetically modified microbes, fungi, plants and animals. The applications of biotechnology include therapeutics, diagnostics, genetically modified crops for agriculture, processed food, bioremediation, waste treatment, and energy production. Three critical research areas of biotechnology are:
(i)Providing the best catalyst in the form of improved organism usually a microbe or pure enzyme.
(ii)Creating optimal conditions through engineering for a catalyst to act, and
(iii)Downstream processing technologies to purify the protein/organic compound.
Let us now learn how human beings have used biotechnology to improve the quality of human life, especially in the field of food production and health.

Applications in Agriculture
∙Agricultural biotechnology is the area of biotechnology involving applications to agriculture.
∙Agricultural biotechnology has been practiced for a long time, as people have sought to improve agriculturally important organisms by selection and breeding.
∙An example of traditional agricultural biotechnology is the development of disease-resistant wheat varieties by cross-breeding different wheat types until the desired disease resistance was present in a resulting new variety.
∙Food production can be increased by:
- i)agro-chemical based agriculture
- ii)organic agriculture
iii)genetically engineered crop-based agriculture
∙Green revolution resulted in great increase in production of food grains (as rice and wheat) due to the introduction of high-yielding varieties, use of pesticides to better management techniques and use of agrochemical (fertilizers and pesticides).
∙For farmers in the developing world, agrochemicals are often too expensive. Further increases in yield with existing varieties are not possible using conventional breeding.
∙Microbes, plants and animals whose genes have been altered by manipulation are called “Genetically Modified Organisms (GMO)”.
∙USES OF GM PLANTS - GM plants have been useful in many ways.
Genetic modification has:
- i)made crops more tolerant to abiotic stresses (cold, drought, salt, heat, etc.)
- ii)reduced reliance on chemical pesticides (pest-resistant crops)
iii)helped to reduce post harvest losses
- iv)increased efficiency of mineral usage by plants (this prevents early exhaustion of fertility of soil).
- v)enhanced nutritional value of food, e.g., Vitamin ‘A’ enriched rice
∙GM has been used to create tailor-made plants to supply alternative resources to industries, in the form of starches, fuels and pharmaceuticals.
∙The production of pest resistant plants, could decrease the amount of pesticide used.
∙Bt toxin is produced by a bacterium called Bacillus thuringiensis (Bt for short).
Bt toxin is produced by cloning the gene responsible for it from the bacteria and expressing the same in plants to provide resistance to insects, without the need for insecticides; this in effect created a bio-pesticide. e.g., Bt cotton, Bt corn, rice, tomato, potato and soyabean.
∙Foods derived from transgenic plants have been called “GMO foods,” “GMPs” (genetically modified products), and “biotech foods.”
∙While some refer to foods developed from genetic engineering technology as “biotechnology-enchanced foods,” others call them “Franken foods.”
Bt Cotton
Insect resistant plants can also be developed by RDT. Progress in engineering insect resistance features in transgenic plants has been made through the expression of insect toxin gene of Bacillus thuringiensis in plants. Most strains of this toxin gene are toxic to lepidopteran insect larvae. The toxicity is due to toxin gene named as Bt - 2.
Some strains of Bacillus thuringiensis produce proteins that kill certain insects such as
- Lepidopterans – e.g. Tobacco budworm, armyworm
- Coleopterans (beetles) and
- Dipterans – e.g. Flies, mosquitoes
- thuringiensis forms certain protein crystals during a particular phase of their growth. These crystals contain a toxic insecticidal protein.
The Bt toxin protein does not cause any damage to the bacterium as it exist as inactive protoxins within the Bacillus, but once an insect ingest the inactive toxin, it is converted into an active form of toxin due to the alkaline pH of insect gut which solubilise the crystals. The activated toxin binds to the surface of mid gut epithelial cells and create pores that cause cell swelling and lysis and eventually cause death of the insect.
Bt Toxin –
Specific Bt toxin genes were isolated from Bacillus thuringiensis and incorporated into the several crop plants such as cotton. The choice of genes depends upon the crop and the targeted pest, as most Bt toxins are insect-group specific. The toxin is coded by a gene named as cry. There are a number of them, for example, the proteins encoded by the genes cryIAc and cryIIAb control the cotton bollworms, whereas that of cryIAb controls corn borer. The protein formed by gene cry II Ab controls the Colorado potato beetle and Cry III Bb controls corn root worm.
Cry genes & their targets
|
Cry Genes |
Target Insects |
|
CryIA – CryIE |
Lepidoptera |
|
CryIIA |
Lepidoptera & Diptera |
|
CryIIB, CryIVA – CryIVD |
Diptera |
|
CryIIIA – CryIIIC |
Coleoptera |
|
CryV, CryVI |
Nematodes |
Bt toxin gene has been cloned from the bacteria and expressed in plants to provide resistance from insects without the requirement of insecticides. It has created a type of biopesticide e.g. Bt cotton, Bt corn, rice, tomato, potato and soyabean etc.
B. thuringiensis produces some types of protein crystals in specific stage of their growth and development. The gene encoding cry protein i.e., cry gene has been isolated and transferred into several crops. This is case where transgene product directly produces the phenotype of interest.

Transgenic plants: Resistance against bacterial and fungal pathogens
Several examples are now available, where genes imparting resistance against bacterial and fungal pathogens have been successfully transferred to tomato (against Pseudomonas, Alternaria and Rhizoctonia) and potato (against Phytophthora).
Transgenic tomato plants for hard skin and improved flavor:
In tomato using antisense RNA technology, transgenic plants have been produced which are either ‘bruise resistant’ (suitable for transport and storage) or exhibit ‘delayed ripening’ giving more time for ripening on the plant, thus permitting more time for sugar accumulation and also giving higher shelf life. These are given the name ‘Flavr Savr’ tomato.
Pest Resistant Plants (RNA interference Method)
Several nematodes parasitizes a wide variety of plants and animals including human beings. A root-knot nematode, Meloidogyne incognita is an important pest of vegetables, cereals, ornamentals, pasture, trees and shrubs, sugarcane, tobacco, cotton, potatoes, etc. It attacks almost all the cultivated plants and can cause high losses. A technique to prevent this infestation is based on the process of RNA interference (RNAi).
RNA interference (RNAi) is a process which involves the introduction of double-stranded RNA into a cell inhibiting the expression of genes. RNAi takes place in all eukaryotic organisms as a method of cellular defense.
Process of RNA interference - This method involves silencing of a specific mRNA due to a complementary ds RNA molecule that binds to and prevents translation of the mRNA (silencing).The complementary RNA could be obtained from viruses having RNA genomes or mobile genetic elements (transposons) that replicate via an RNA intermediate.
Using Agrobacterium vectors, nematode-specific genes were introduced into the host plant. The introduction of DNA was such that it produced both sense and anti-sense RNA in the host cells. These two RNA’s being complementary to each other formed a double stranded (ds RNA) that initiated RNAi and thus, silenced the specific mRNA of the nematode. The consequence was that the parasite could not survive in a transgenic host expressing specific interfering RNA. The transgenic plant therefore got itself protected from the parasite.
The Noble Prize in Physiology or Medicine 2006 was awarded jointly to Andrew Z. Fire and Craig C. Mello for their discovery of RNA interference-gene silencing by double-stranded RNA.
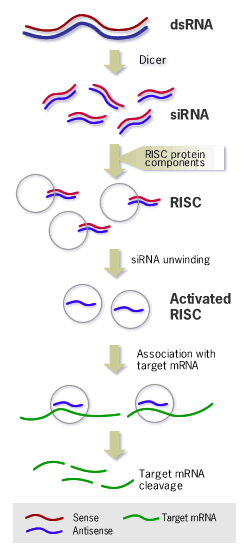
The Mechanism of RNA Interference (RNAi)
Long double-stranded RNAs (dsRNAs; typically >200 nt) can be used to silence the expression of target genes in a variety of organisms and cell types (e.g., worms, fruit flies, and plants).
Upon introduction, the long ds RNAs enter a cellular pathway that is commonly referred to as the RNA interference (RNAi) pathway.
First, the ds RNAs get processed into 20-25 nucleotide (nt) small interfering RNAs (siRNAs) by an RNase III-like enzyme called Dicer (initiation step).
Then, the siRNAs assemble into endoribonuclease - containing complexes known as RNA-induced silencing complexes (RISCs), unwinding in the process.
The siRNA strands subsequently guide the RISCs to complementary RNA molecules, where they cleave and destroy the cognate RNA (effecter step). Cleavage of cognate RNA takes place near the middle of the region bound by the siRNA strand. In mammalian cells, introduction of long dsRNA (>30 nt) initiates a potent antiviral response, exemplified by nonspecific inhibition of protein synthesis and RNA degradation. The mammalian antiviral response can be bypassed, however, by the introduction or expression of siRNAs
BIOTECHNOLOGICAL APPLICATIONS IN MEDICINE
The recombinant DNA technological processes have made immense impact in the area of healthcare by enabling mass production of safe and more effective therapeutic drugs. Further, the recombinant therapeutics do not induce unwanted immunological responses as is common in case of similar products isolated from non-human sources. At present, about 30 recombinant therapeutics have been approved for human-use the world over. In India, 12 of these are presently being marketed.
Genetically Engineered Insulin
Insulin is a protein hormone made in the pancreas which plays a vital role in the regulation of blood sugar levels. Its deficiency is one of the causes of the disease diabetes mellitus (sugar diabetes) where blood sugar levels become raised with harmful consequences.
Daily injections of insulin isolated from the pancreas of slaughtered pigs and cattle became the standard treatment. However, due to minor differences in the amino acid composition of insulin from species different to ourselves, and to traces of impurities, some patients were allergic to animal insulin and showed damaging side effects as a result of the injections. The ideal solution became possible with the introduction of genetic engineering. The gene for human insulin is inserted into a bacterium, and the bacterium is grown in a fermenter to make large quantities of the protein. An outline of the procedure currently used for human insulin production is shown in figure.
.jpg)
Insulin consists of 51 amino acids forming two short polypeptide chains - chain A having 21 amino acids and chain B with 30 amino acids. The two chains are linked by disulfide bond. In animals, including humans, insulin occurs as proinsulin. It is made of chain A, chain B and chain C (30 amino acids). As the insulin matures, chain C is removed.
The genetic engineering of insulin begins with identification and separation of DNA sequences coding for chain A and chain B. This was found to be present at the top of the short arm of the eleventh chromosome. It contains 153 nucleotides-63 nucleotides for chain A and 90 nucleotides for chain B.
The expression of genes for human insulin A and B polypeptide chains in E. coli. The two genes are expressed in separate bacterial systems. The A and B chains are then refolded and joined covalently to form the mature protein, insulin.
These sequences were introduced into plasmid (pBR322) of Escherichia coli - common human colon bacterium. It is said to be the factory used in genetic engineering of insulin. In E. coli, -galactosidase controls the transcription of these genes, therefore, insulin gene needs to be tied to this enzyme. The protein formed by E. coli consists partly of -galactosidase joined to either A or B chain of insulin.
These are then extracted from -galactosidase fragment and purified. The two chains are mixed and reconnected in a reaction that forms disulfide bridges resulting in pure humulin-the synthetic human insulin.
DEVELOPMENT OF HUMULIN –
The original technique was developed by Eli Lilly and Company and in 1982 human insulin, marketed as ‘humulin’, became the first genetically engineered pharmaceutical product to be approved for use.
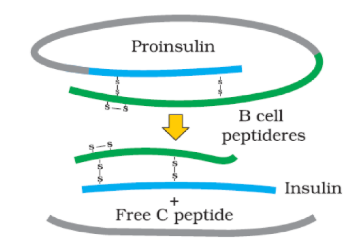
Maturation of pro-insulin into insulin after removal of Cpeptide (to be simplified)