Acids and Bases is one of the most important chapters in Class 9 Science. It introduces key concepts such as pH, neutralization, and the role of acids and bases in daily life. Our NCERT solutions for Class 9 Science provide stepwise answers and experiments that make chemical reactions easy to grasp. The class 9 notes simplify equations, indicators, and laboratory tests. With the help of class 9 science tuition, students can understand how acids and bases react with metals, carbonates, and oxides. Tutors conduct interactive sessions and simple experiments to make chemistry fun. Enrolling in Class 9 tuitions ensures personalized doubt-solving and exam-focused preparation. Learning with expert tutors builds confidence in understanding the chemical behavior of substances, enhancing both academic scores and curiosity.
Acids & Bases are two important classes of compounds which we come across in chemistry. Initially, the substances which are sour to taste were termed Acids. In fact, the name acid was derived from a Latin word 'acidum'. Similarly, the Bases or alkalis were characterized by their bitter taste and their slippery touch.
Salt is another important category of chemical compounds. The term 'salt' covers all electrovalent compounds having positive & negative radicals formed by the reactions of corresponding acids and bases.
Arrhenius Concept (Water Ion System)
According to this concept, an acid is any hydrogen containing compound which gives H+ ions in aqueous solution and a base which gives OH- ions in aqueous solution. Thus HCl is an acid and NaOH is a base and the neutralization process can be represented by a reaction involving the combination of H+ and OH- ions to form H2O.
HCl ⇌ H+ + Cl- (in H2O)
NaOH ⇌ Na+ + OH- (in H2O)
Where, H+ + OH- → H2O
The main points of this theory are:
- Upon dilution, the ions get separated from each other. This is known as the dissociation of ions.
- The fraction of the acid or base which dissociates into ions is called its degree of dissociation and is denoted by alpha(α) which can be calculated by the following formula:
α = No. of molecules dissociated at equilibrium / total no. of molecules
- The degree of dissociation depends upon the nature of an acid or a base. Strong acids and bases are highly dissociated, while weak acids and bases are dissociated to a lesser extent
- An electric current is carried by the movement of ions. Greater the ionic mobility more will be the conductivity of the acid or base.
- H+ ions do not exist as such but exist in combination with molecules of H2O and H3O+ ions (known as hydronium ion).
H+ + H2O ⇌ H3O+
Arrhenius Acids: HF, HCl, HBr, HI, H2SO4, HNO3 etc.
Arrhenius Bases: NaOH, KOH, Mg(OH)2, Ca(OH)2, Al(OH)3 etc.
Bronsted - Lowry Theory (Proton - donor - Acceptor System)
Bronsted and Lowry in 1923 independently proposed a more general definition of acids and bases. According to them, an acid is defined as any hydrogen containing material (a molecule or an ion) that can release a proton (H+) to any other substance, whereas a base is any substance (a molecule or an ion) that can accept a proton from any other substance. In short, an acid is a proton-donor and a base is a proton-acceptor.
Example: HCl + H2O ⇌ H3O+ + Cl-
In this reaction, HCl acts as an acid because it donates a proton to the water molecule. Water on another hand, behaves as a base by accepting a proton.
Conjugate Acid - Base Pairs
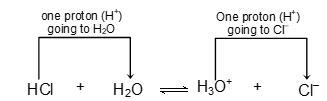
In this reaction HCl donates a proton to H2O, therefore HCl is a Bronsted acid. Water, on the other hand, accepts a proton from HCl, and is, therefore, a Bronsted base. In the reverse reaction which at equilibrium proceeds at the same rate as the forward reaction, the H3O+ ions donates a proton to Cl- ion, hence H3O+ ion is a Bronsted acid and Cl- ion, because it accepts a proton from H3O+ ion, is a Bronsted base.
HCl ⇌ Cl- + H+ and H3O+ ⇌ H2O + H+
The members of which can be formed from each other mutually by the gain or loss of proton are called conjugate acid - base pairs. If in the below mentioned reaction, the acid HCl is labeled Acid1 then Cl- is its conjugate base designated Base1 and if H2O is designated as Base2 then H3O+ is its conjugate acid designated as Acid2. The equilibrium can be represented by a general equation:
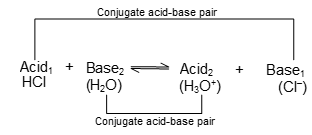


This is the fundamental equation representing the relationship between an acid and a base on the basis of Bronsted-Lowry concept. Thus on the basis of this concept, Acid1 and Base1 form one conjugate acid-base pair and Acid2 and Base2 form another conjugate acid-base pair.
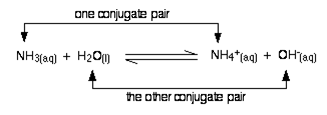
The species like H2O, NH3, which can act as both acid and base are called amphiprotic. Moreover according to the theory, an acid on losing a proton becomes a base, called conjugate base, while the base by accepting proton changes to acid called conjugate acid.
Examples:
- H2SO4 + H2O → HSO4- + H3O+
- HSO4- + NH3 → SO42- + NH4+
- HCl + NH3 → Cl- + NH4+
- HCN + H2O → H3O+ + CN-
Lewis Concept (Electron - Donor - Acceptor System)
This theory explains the acid-base phenomena not in terms of ionic reactions but in terms of electronic structure of the acid and base along with the formation of a coordinate covalent bond. According to Lewis (1923), an acid is any species (molecule, radical or ion) that can accept an electron-pair to form a coordinate covalent bond and a base is any species that can donate an electron-pair to the formation of a coordinate covalent bond. Thus, in the Lewis system, an acid is an electron pair-acceptor and a base is an electron pair-donor.
Thus according to Lewis theory, the process of neutralization is simply the formation of a coordinate bond between an acid and a base. The neutralization product, termed as coordinate complex or adduct, may be either non-ionisable or may undergo dissociation or condensation reaction depending on its stability.
Now consider the reaction between a proton (H+) and :NH3 molecules as shown below.
H+ (proton) + :NH3 → (H←NH3)+
Acid + Base → Adduct
Evidently in the above reaction proton (H+) accepts one electron pair from :NH3 molecule and is therefore, an acid, whereas :NH3 molecule which donates an electron pair, is a base. The adduct is NH4+ ion.
Lewis bases and Bronsted-Lowry bases are the same substances, since any molecule or ion which accepts protons does so because of the presence of an unshared pair of electrons. In the above example NH3 molecules is a proton acceptor (i.e., Bronsted-base) and an electron pair donor (i.e., Lewis - base). Bronsted and Lewis theories are thus identical as far as bases are concerned except that the wording used for definition of the bases is different in both the theories.
Thus NH3, H2O, OH-, Cl-, CN- etc. are the bases on the Bronsted as well as on the Lewis systems. There are however, few compounds such as amides, ethers, nitriles, C2H4, C2H2, C6H6 etc. which have little or no tendency to accept protons but react readily with Lewis - acids.
Limitations:
- This theory failed to explain the acidic and basic nature of general acids and bases.
- This theory could not explain the general neutralization reactions involving the formation of salts and water.
- Generally, the acid-base reactions are fast reactions. But the formation of a coordinate bond is a slow process.
- This Theory cannot explain the relative strengths of acids and bases.
Utility of Lewis Concept:
- This concept also includes those reactions in which no protons are involved.
- Lewis concept is more general than the Bronsted-Lowry concept in that acid-base behavior is not dependent on the presence of one particular element or on the presence or absence of a solvent.
- It explains the long accepted basic properties of metallic oxides and acidic properties of non-metallic oxides.
- This theory also includes many reactions such as gas phase, high temperature and non-solvent reaction as neutralization process.
- The Lewis approach is however of great value in case where the protonic concept is inapplicable. For example, in reaction between acidic and basic oxides in the fused state.
Lewis Acids: H+, Cu2+, Fe2+, Fe3+, BF3, AlF3
Lewis Bases: OH-, CN-, CH3COO-, :NH3, H2O:
Acids
Substances with sour taste are regarded as acids. Lemon juice, Vinegar, grape fruit juice and spoilt milk etc., taste sour since they are acidic. Many substances can be identified as acids based on their taste, but some of the acids like sulphuric acid have very strong action on the skin which means that they are corrosive in nature. In such cases it would be dangerous to taste them.
According to the modern definition: An acid may be defined as a substance which releases one or more H+ ions in aqueous solution.
Acids are mostly obtained from natural sources. On the basis of their source acids are of two types:
Mineral Acids:
Acids which are obtained from rocks and minerals are called mineral acids. For Example HCl, H2SO4, HNO3 etc.
Organic acids
Acids which are present in animals and plants are known as organic acids. For example formic acid (HCOOH), acetic acid (CH3COOH) etc.
Methods of Preparation of Acids
By synthesis/direct combination (for hydracids and binary acids)
- Hydrogen + Non-metal → Acid
- H2 + Cl2 → 2HCl (sunlight)
- H2 + Br2 → 2HBr (sunlight)
- H2 + I2 → 2HI
By the reaction of non-metallic (acidic) oxides with water.
- Non-metallic oxide + Water → Acid
- SO3 + H2O → H2SO4
- P2O5 + 3H2O → 2H3PO4
- N2O5 + H2O → 2HNO3
By the oxidation of non- metals with oxy acids
- S + 6HNO3 → H2SO4 + 2H2O + 6NO2
- 2P + 5H2SO4 → 2H3PO4 + 5SO2 + 2H2O
By the displacement of salts of more volatile acids with less volatile acid.
- Salt + Less volatile acids → Salt + more volatile acid
- NaCl + H2SO4 → NaHSO4 + HCl
- NaNO3 + H2SO4 → NaHSO4 + HNO3
Chemical Properties of Acids
1. Action with metals:
Dilute acids like dilute HCl and dilute H2SO4 react with certain active metals to evolve hydrogen gas.
2Na(s) + 2HCl (dil) → 2NaCl(aq) + H2(g)
Mg(s) + H2SO4 (dil) → MgSO4(aq) + H2(g)
Metals which can displace hydrogen from dilute acids are known as active metals. E.g. Na, K, Zn, Fe, Ca, Mg etc.
Zn(s) + H2SO4 (dil) → ZnSO4(aq) + H2(g)
The active metals which lie above hydrogen in the activity series are electropositive in nature. Their atoms lose electrons to form positive ions and these electrons are accepted by H+ ion of the acid. As a result, H2 is evolved.
Example:
Zn(s) → Zn2+(aq) + 2e-
2H+(aq) + SO42-(aq) + 2e- → H2(g) + SO42-(aq)
Zn(s) + 2H+(aq) → Zn++(aq) + H2(g)
2. Action with metal oxides:
Acids react with metal oxides to form salt and water. These reactions are mostly carried out on heating.
Example:
ZnO(s) + 2HCl(aq) → ZnCl2(aq) + H2O(l)
MgO(s) + H2SO4(aq) → MgSO4(aq) + H2O(l)
CuO(s) + 2HCl(aq.) → CuCl2(aq) + H2O (l)
3. Action with metal carbonates and metal bicarbonates:
Both metal carbonates and bicarbonates react with acids to evolve CO2 gas and form salts.
Example:
CaCO3(s) + 2HCl(aq) → CaCl2(aq) + H2O(l) + CO2(g)
2NaHCO3(s) + H2SO4(aq) → Na2SO4(aq) + 2H2O(aq) + 2CO2(g)
4. Action with bases:
Acids react with bases to give salt and water.
HCl + NaOH → NaCl + H2O
Classification of Acids
On the basis of strength
- Strong acids: The acids which undergo nearly 100% ionization in aqueous solutions are called strong acids. These produce a high concentration of H+ ions in the solution.
Example: HCl (aq) → H+(aq) + Cl- (aq) (100% ionized) - Weak acids: Acids which undergo partial ionization in aqueous solutions are called weak acids. These produce low concentration of H+ ions in the solution.
Example: CH3COOH(aq) ⇌ CH3COO- (aq) + H+(aq)
On the basis of basicity
Number of H+ or H3O+ ions produced by ionization of one molecule of an acid in aqueous solution is called its basicity.
- Monobasic acids: The acids which dissociate in aqueous solutions to produce one hydronium ion per molecule of the acid are called monobasic acids.
Example: HCl (aq) → H+(aq) + Cl- (aq) - Dibasic acids: The acids which dissociate in aqueous solution to produce two hydronium ions per molecule of the acids are called dibasic acids.
Example: H2SO4 (aq) → 2 H+(aq) + SO4-2 (aq) - Tribasic acids: The acids which dissociate in aqueous solution to produce three hydronium ions per molecule of acids are called tribasic acids.
Example: H3PO4 (aq) → 3 H+(aq) + PO43-(aq)
On the basis of concentration
- Concentrated acid: An aqueous solution that has a high percentage of acid and a low percentage of water is said to be a concentrated acid.
- Dilute acid: An aqueous solution that has a low percentage of acid and a high percentage of water is said to be a dilute acid.
Base
Substances with bitter taste and soapy touch are regarded as bases. Many bases like sodium hydroxide and potassium hydroxide have corrosive action on the skin and can even harm the body. A base may be defined as a substance capable of releasing one or more OH- ions in aqueous solution.
Some bases like sodium hydroxide and potassium hydroxide are water soluble. These are known as alkalis. Therefore water soluble bases are known as alkalis eg. KOH, NaOH. Bases like Cu(OH)2, Fe(OH)3 and Al(OH)3 are not alkalis. Most metallic oxides are basic in nature since these react with water to form basic solutions (hydroxides) and can react with acids directly to form salts.
Methods of Preparation
By the action of water on metals
- Water + Metal → Base/Alkali + Hydrogen
- 2H2O(l) + 2Na → 2NaOH + H2
- H2O(g) + Mg → MgO + H2
- 4H2O(g) + 3Fe ⇌ Fe3O4 + 4H2
By the action of metallic oxides on water
- Metallic oxide + Water → Base
- K2O + H2O → 2KOH
- MgO + H2O → Mg(OH)2
By the action of oxygen on metals
- Metal + Oxygen → Metallic oxides
- 2Mg + O2 → 2MgO
- 4Fe + 3O2 → 2Fe2O3
By the decomposition of metal carbonates
- Metal carbonate → Basic oxide + Carbon dioxide
- ZnCO3 → ZnO + CO2
- CuCO3 → CuO + CO2
Using soluble metal salts and NaOH/KOH
- Soluble metal salt + NaOH/KOH → Salt + Insoluble metal hydroxide
- AlCl3 + 3NaOH → 3NaCl + Al(OH)3
- Zn(NO3)2 + 2KOH → 2KNO3 + Zn(OH)2↓
Strong heating of metal nitrates
- Metal nitrate → Metal oxide + NO2 + O2
- 2Ca(NO3)2 → 2CaO + 4NO2 + O2
- 2Zn(NO3)2 → 2ZnO + 4NO2 + O2
By the action of oxygen on metal sulphides
- Metal sulphide + Oxygen → Metal oxide + Sulphur dioxide
- 2ZnS + 3O2 → 2ZnO + 2SO2
- 2Cu2S + 3O2 → 2Cu2O + 2SO2
Chemical Properties
Action with metals:
Metals like zinc, tin and aluminium react with strong alkalis like NaOH (caustic soda), KOH (caustic potash) to evolve hydrogen gas.
Zn(s) + 2NaOH (aq) → Na2ZnO2(aq) + H2(g)
(Sodium zincate)
Sn(s) + 2NaOH(aq) → Na2SnO2(aq) + H2(g)
(Sodium stannite)
2Al(s) + 2NaOH + 2H2O → 2NaAlO2(aq) + 3H2(g)
(Sodium metaaluminate)
Action with non-metallic oxides:
Acids react with metal oxides, but bases react with oxides of non-metals to form salt and water.
Example:
2NaOH(aq) + CO2(g) → Na2CO3(aq) + H2O(l)
Ca(OH)2(s) + SO2(g) → CaSO3(aq) + H2O(l)
Ca(OH)2(s) + CO2(g) → CaCO3(s) + H2O(l)
Classification of Bases
On the basis of strength
- Strong bases: Bases which undergo nearly 100% (Complete) ionization in aqueous solutions. These produce high concentration of OH- ions in the solution.
Example: NaOH (aq) → Na+(aq) + OH-(aq) (100% ionized) - Weak Bases: Bases which undergo partial ionization in aqueous solutions. It produces low concentration of OH- ions in the solution.
Example: NH4OH ⇌ NH4+ + OH-
On the basis of acidity
Number of OH- ions produced by the ionization of one molecule of base in aqueous solution is called its acidity.
- Monoacidic bases: The bases which dissociate in aqueous solution to produce one hydroxyl ion per molecule of the base is called monoacidic bases.
Example: NaOH, KOH, NH4OH etc. - Diacidic bases: The bases which dissociate in aqueous solutions to produce two hydroxyl ions per molecule of the base are called Diacidic base.
Example: Ca(OH)2, Cu(OH)2, Mg(OH)2 etc - Triacidic bases: Triacidic bases are those which dissociate in aqueous solutions to produce three hydroxyl ions per molecule of base.
Example: Al(OH)3, Fe(OH)3 etc
Role of Water in the Ionisation of Acids and Bases
Substances can act as acids or bases only in the presence of water (in aqueous solution). In dry state which is also called anhydrous state, these characters cannot be shown. Actually, water helps in the ionisation of acid or dissociation and it is explained on the basis of a theory called Arrhenius theory of acids and bases. In the dry state, hydrochloric acid is known as hydrogen chloride gas i.e. HCl(g).
It is not in the position to give any H+ ions. Therefore, the acidic character is not shown. Now, let us pass the gas through water taken in a beaker with the help of glass pipe. H2O molecules are of polar nature which means that they have partial negative charge (δ-) on oxygen atom and partial positive charge (δ+) on hydrogen atoms. They will try to form a sort of envelope around the hydrogen atoms as well as chlorine atoms present in the acid and thus help in their separation as ions. These ions are said to be hydrated ions.
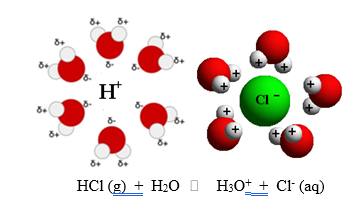


Electrical current is carried through these ions. The same applies to other acids as well as bases. Thus we conclude that:
- Acids can release H+ ions only in aqueous solution.
- Bases can release OH- ions only in aqueous solution
- Hydration helps in the release of ions from acids and bases.
Dilution of Acids & Bases
Acids and bases are mostly water soluble and can be diluted by adding the required amount of water. With the addition of water the amount of acids or base per unit volume decreases and dilution occurs. The process is generally exothermic in nature when a concentrated acid like sulphuric acid or nitric acid is to be diluted with water, acid should be added drop wise to water taken in the container with constant stirring.
Conducting Nature of Acid and Base Solution
Acids are the substances which contain one or more hydrogen atoms in their molecules which they can release in water as H+ ions. Similarly, bases are the substances which contain one or more hydroxyl groups in their molecules which they can release in water as OH- ions. Since the ions are the carrier of charge therefore, the aqueous solutions of both acid and bases are conductors of electricity.
Neutralization Reaction
It is the reaction of acid and base to form salt & water and is associated with liberation of heat. The relative quantities of acids & bases undergoing neutralization reaction and hence the heat liberated depends upon the concentration of H+ ions & OH- ions produced by the dissociation of acid or base in a given reaction. Since the acids and bases differ in their basicities & acidities, the concentration of H+ & OH- ions furnished by them also differs.
Acid + Base → Salt + water + heat
Heat of neutralization
The amount of heat liberated when one equivalent of an acid reacts with one equivalent of a base. For any strong acid-strong base reaction, the heat of neutralization has the same value i.e., 13.7 kcal/mole since the acid and base involved are completely ionized.
pH Scale
The acidic or the basic strength of a solution is ascertained in on a scale, known as the pH scale, which gives a value called pH value. The p in pH stands for 'Potenz' in German meaning power. The pH of a solution is the negative logarithm to the base 10 of the hydrogen ion concentration expressed in moles per litre. For example, a solution of H+ ions of concentration equal to 10-12 moles/L will have pH = 12.
pH = - log10 [H+]
The normal pH scale ranges from 0 to 14, as given
The number 7 on the pH scale represents the neutrality of the solution. Numbers less than 7, i.e. pH 6,5,4,....... indicate acidity increasing as the numbers decrease; numbers greater than 7, i.e. pH 8,9,10,....... indicate alkalinity increasing as the numbers increase.
In a colourless liquid, a reasonably accurate pH value can be obtained by addition of a universal indicator. The universal indicator is a mixture of organic dyes or of several indicators. It shows different colours at different concentration of hydrogen ions in a solution.
A universal indicator produces a green colour in a neutral solution progressively from blue to indigo to violet as pH increases progressively from 7 to 14. The colour change in acidic solution is from yellow to pink, and then to red, as pH progressively decreases from 7 to 1.
Indicators
An indicator indicates the nature of a particular solution whether acidic, basic or neutral. Apart from this, indicator also represents the change in nature of the solution from acidic to basic and vice versa. Indicators are basically coloured organic substances extracted from different plants. A few common acid base indicators are.
| Indicator | Description |
|---|---|
| Litmus | Litmus is a purple dye which is extracted from 'lichen' a plant belonging to variety Thallophyta. It can also be applied on paper in the form of strips and is available as blue and red strips. A blue litmus strip, when dipped in an acid solution acquires red colour. Similarly a red strip when dipped in a base solution becomes blue. |
| Phenolphthalein | It is also an organic dye and acidic in nature. In neutral or acidic solution, it remains colourless while in the basic solution, the colour of indicator changes to pink. |
| Methyl Orange | Methyl orange is an orange or yellow coloured dye and basic in nature. In the acidic medium the colour of indicator becomes red and in the basic or neutral medium, its colour remains unchanged |
| Red Cabbage Juice | It is purple in colour in neutral medium and turns red or pink in the acidic medium. In the basic or alkaline medium, its colour changes to green. |
Salts
Salts are the ionic compounds consisting of two parts, one part carrying a positive charge (called positive ion or cation) and the other part carrying a negative charge (called a negative ion or anion). The number of positive and negative ions present is such that total positive charge is equal to total negative charge so the salt as a whole is the electrically neutral. The positive ion is generally a metal ion, the most common exception being that of ammonium ion, NH4+.
A large number of reactions take place forming salts. A few most common reactions are given below:
1. By neutralization of acids and bases:
Acids react with bases to form salt and water.
For example
NaOH + HCl → NaCl + H2O
The positive part of the salt which comes from the base is called basic radical whereas the negative part of the salt which comes from the acid is called acid radical. For example, in the salt NaCl, Na+ come from the base, NaOH and is the basic radical whereas Cl- which comes from the acid, HCl, is the acid radical.
2. By action of metals on acids:
Active metals react with the acids forming salt and hydrogen gas.
For Example:
Zn + H2SO4 → ZnSO4 + H2
3. By action of acids on metal carbonates and bicarbonates
For example,
CaCO3 + 2HCl → CaCl2 + H2O + CO2
4. By action of metals on alkalies:
Metals like zinc and aluminium react with alkalies sodium hydroxide and potassium hydroxide on heating to form salt and give out hydrogen gas. For example,
2NaOH + Zn → Na2ZnO2 + H2
Sodium zincate ion formed is a salt consisting of the positive sodium ion (Na+) and the negative zincate ion (ZnO2-)
Families of Salts
As already explained, the most common method of formation of salts is by the reaction between an acid and a base. The salts are classified into different families either on the basis of the acid or on the basis of the base from which they have been obtained.
- Chlorides: The salts which are formed by reaction of hydrochloric acid (HCl) with any base are called chlorides.
- Nitrates: The salts which are formed by reaction of nitric acid (HNO3) with any base are called nitrates.
- Sulphates: The salts which are formed by reaction of sulphuric acid (H2SO4) with any base are called sulphates.
- Carbonates: The salts which are formed from carbonic acid (H2CO3) are called carbonates.
Normal Salts and Acidic Salts
If all the ionizable ions of a polyprotic (or polybasic) acid are replaced by the metal ions (or ammonium ions), the salts obtained are called normal salts. However, if all the ionizable hydrogen ions are not replaced, i.e., some acidic hydrogen ions still remain, the salt obtained acidic salt. Thus polyprotic acids can form a series of salts.
For example,
- Sulphuric acid (H2SO4) is diprotic. It can form the normal salts like Na2SO4, K2SO4 etc. or it can form acidic salts like NaHSO4, KHSO4 etc. These are called hydrogen sulphates or bisulphates. Thus, NaHSO4 is sodium hydrogen sulphate or sodium bisulphate.
- Carbonic acid (H2CO3) is also diprotic. It can form normal salts like Na2CO3, K2CO3 etc. or acidic salts like NaHCO3. KHCO3 etc. These are called hydrogen carbonates or bicarbonates. For example, NaHCO3 is called sodium hydrogen carbonate or sodium bicarbonate.
Similarly, when a base is not completely neutralized by the acid, the salt obtained is called basic salt, e.g., basic magnesium chloride, Mg(OH)Cl.
Water of Crystallization
Some salts, while crystallizing out from their solutions, unite with a definite quantity of water, which is known as the water of crystallization. The water of crystallization is in loose chemical combination with the salts, and it can be driven out by heating the powdered crystals of these salts above 100°C.
Laboratory Preparation of Some Salts
1. Iron chloride [FeCl3]
Heated iron powder reacts directly with dry chlorine gas to produce anhydrous iron (III) chloride, which sublimates and is collected as a condensate
2Fe + 3Cl2 → 2FeCl3 (heated, dry)
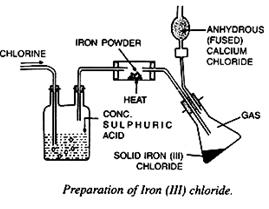
Procedure:
-
Powdered iron is taken in a combustion tube. Dry chlorine gas which is obtained by passing the gas through heated concentrated sulphuric acid is then passed through the combustion tube. The air inside the apparatus is expelled.
-
The iron turns red hot, since the reaction is exothermic. Now, external heating is suspended.
-
Iron (III) chloride is formed which volatilizes and condenses in the receiver as brown scale.
-
Iron (III) chloride is highly deliquescent, so it is kept dry with the help of fused calcium chloride (drying agent).
2. Copper (II) sulphate (or blue vitriol)
Insoluble hydroxides, and basic oxides, react with dilute acids to give their corresponding salts.
Cu(OH)2 + H2SO4 → CuSO4 + 2H2O
CuO + H2SO4 → CuSO4 + H2O
CuSO3 + H2SO4 → CuSO4 + H2O + CO2
CuSO4 + 5H2O → CuSO4.5H2O (blue vitriol)
Procedure:
-
Take dilute sulphuric acid in a beaker and heat it on wire gauze.
-
Add copper hydroxide or black cupric oxide or copper carbonate, in small quantities at a time, with stirring, till no more of it dissolves and the excess compound settles to the bottom.
-
Filter it hot, and collect the filtrate in a china dish. Evaporate the filtrate by heating to the point of crystallization, and then allow it to cool.
-
Collect the bright blue crystals of copper (II) sulphate penta-hydrate CuSO4.5H2O (blue vitriol) and dry the crystals.
3. Zinc sulphate (or white vitriol)
Zn(s) + H2SO4(aq) → ZnSO4 + H2
ZnSO4 + 7H2O → ZnSO4.7 H2O (white vitriol)
Procedure
-
Take dilute sulphuric acid (1 volume of acid: 5 volumes of water) in a beaker and heat it on a wire gauze. Add some granulated zinc pieces, a little at a time, with constant stirring.
-
Add zinc till the metal settles at the base of the beaker.
-
Effervescence takes place because of the liberation of hydrogen gas. If the reaction is too slow, a few drops of copper sulphate solution (catalyst) are added to make it proceed faster. When effervescence stops, it indicates that all the acid has been used up.
-
The excess of zinc is filtered off.
-
Collect the solution in a china dish. Evaporate the solution. Crystals appear, filter and wash them with water and dry them between the folds of a filter paper.
-
The white, needle-shaped crystals are of hydrated zinc sulphate, commonly known as white vitriol.
4. Iron (II) sulphate (or green vitriol)
Iron (II) sulphate is prepared by the same method as white vitriol
Fe(s) + H2SO4 → FeSO4 + H2
FeSO4 + 7H2O → FeSO4.7H2O (green vitriol)
Filter the hot solution formed and allow the filtrate to evaporate at room temperature. Pale green crystals of hydrated iron (II) sulphate are formed.
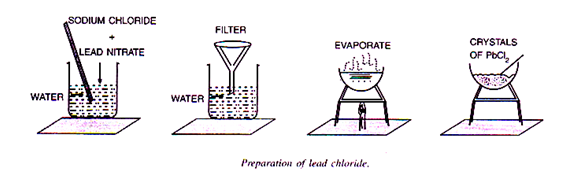
5. Lead chloride (PbCl2)
An insoluble salt can be prepared by mixing the solutions of two appropriate salts, each containing one of its ions. This mixing results in the formation of an insoluble solid precipitate, which can be filtered, washed and dried.
It is prepared by adding either dilute hydrochloric acid or sodium chloride solution to a solution of lead nitrate. A white precipitate of lead chloride slowly appears.
Pb(NO3)2 + 2HCl → PbCl2↓ + 2HNO3
The heavy, white precipitate of lead chloride is filtered and washed repeatedly with cold water. The precipitate is then taken in a china dish with some water. It is heated till the precipitate of lead chloride dissolves. The solution is then cooled.
Pure, needle shaped crystals of lead chloride, are thus obtained.
The chlorides of Pb, Ag and Hg, and the sulphates of Ba, Pb and Ca are also prepared using this method.


6. Calcium Carbonate (CaCO3)
It is prepared by adding sodium carbonate solution to a hot solution of calcium chloride in a beaker, till the former is in excess.
CaCl2 + Na2CO3 → CaCO3↓ + 2NaCl
The white precipitate formed by the interchange of radicals is filtered and washed repeatedly with cold water and collected. The washed precipitate is dried to obtain an amorphous powder. i.e. calcium carbonate.
Some Industrially Important Salts
1. COMMON SALT (NaCl)
Chemically, common salt is sodium chloride, NaCl. Table salt, used as a food material is iodized salt containing a small amount of potassium iodide (> 15 ppm) which protects us form thyroid disorder.
Occurrence and extraction:
- From sea water, by evaporation
- Rock salt formed from dried up seas
- Inland takes due to natural evaporation of water.
Properties
- Colourless, crystalline with melting point 820°C.
- Soluble at room temperature, Solubility does not vary much with temperature.
- On heating, a cracking sound is produced.
- It is hygroscopic. i.e., takes up moisture from air and becomes wet.
- Reacts with concentrated H2SO4 to give out HCl gas.
Uses
- Essential constituent of diet
- Preservation for packed food materials like fish.
- Makes freezing mixture with ice.
- In salting out soap, preparation of pottery glaze etc.
- A raw material for production of other chemical like baking soda, washing soda, caustic soda, chlorine, sodium, bleaching powder.
2. CAUSTIC SODA
Chemically, caustic soda is sodium hydroxide, NaOH.
Manufacture
By electrolysis of an aqueous solution chloride (called brine). The method is called chlor – alkali process because it gives chlorine and the alkali, NaOH. Hydrogen and chlorine are obtained as by - products. They can be combined to produce HCl gas which can be dissolved in water to produce hydrochloric acid.
2 H2O + 2 NaCl → 2 NaOH + H2 + Cl2
2Cl- → Cl2 + 2e-
2 H2O + 2e- → 2 OH- + H2
Uses of caustic soda:
- In making soaps
- For degreasing metals
- Paper, dye and rayon industry
- In petroleum refining
- For mercerizing cotton
- As laboratory reagent.
3. BLEACHING POWDER (CaOCl2)
Chemically, Bleaching powder is calcium oxychloride (CaOCl2).
Manufacture:
Bleaching powder is manufactured by action of chlorine on dry slaked lime.
Ca(OH)2 + Cl2 → CaOCl2 + H2O
Properties of bleaching powder.
- It is yellowish white powder.
- When exposed to air, it reacts with CO2 of the air to liberate Cl2 gas.
- It is soluble in cold water. The milkiness of the solution is due to presence of unreacted lime.
- It reacts with HCl and H2SO4, liberating Cl2 gas (called available chlorine).
Uses of bleaching powder:
- For bleaching cotton in textile industry and wood pulp in paper industry.
- For disinfecting drinking water.
- As an oxidizing agent.
- In manufacture of chloroform.
4. BAKING SODA (NaHCO3)
Chemically, baking soda is sodium hydrogen carbonate (NaHCO3).
Manufacture:
It is prepared by passing CO2 through NaCl solution (brine) saturated with NH3
NaCl + H2O + CO2 + NH3 → NH4Cl + NaHCO3
It can also be prepared by passing CO2 through aqueous Na2CO3 solution.
Properties:
-
It is a white crystalline solid, stable in air, sparingly soluble in water (solution being alkaline).
-
On heating, it decomposes to give Na2CO3 and CO2.
-
It reacts with acids with effervescence to give out CO2 gas.
Uses:
It is used in medicines as antacid, as a constituent of baking powder for preparing fluffy foodstuff like cake, bread, etc and in fire extinguishers.
5. WASHING SODA (SODIUM CARBONATE)
Chemically, washing soda is sodium carbonate decahydrate (Na2CO3.10H2O). Anhydrous sodium carbonate (Na2CO3) is called soda – ash. Sodium carbonate exists in three forms: Na2CO3.10H2O, Na2CO3.H2O and Na2CO3.
Manufacture
Sodium carbonate is manufactured by Solvay process in the following steps:
1. Manufacture of sodium hydrogen carbonate.
By passing CO2 through NaCl solution saturated with NH3
NH3 + H2O + CO2 → NH4HCO3
NaCl + NH4HCO3 → NaHCO3 + NH4Cl
NaHCO3 being less soluble separates out as crystalline solid and is filtered off.
2. Calcination of NaHCO3.
NaHCO3 crystals obtained in step (i) are heated strongly. They decompose to form anhydrous Na2CO3 (soda ash).
2 NaHCO3 → Na2CO3 + H2O + CO2
3. Recrystallization of sodium carbonate.
Anhydrous sodium carbonate is converted into washing soda by dissolving in hot water and then subjecting to recrystallization
Na2CO3 + 10 H2O → Na2CO3.10 H2O
CO2 required is step (i) is obtained from limestone (CaCO3). Hence, the raw materials required are NaCl, NH3 and CaCO3
Properties of washing soda:
- It is a transparent crystalline solid with the formula Na2CO3.10H2O.
- On exposure of air, it decomposes to form monohydrate which is white power. The process is called efflorescence.
- Its aqueous solution in water is alkaline.
- On heating, it forms anhydrous Na2CO3.
- It reacts with acids to evolve CO2 with brisk effervescence.
Uses of sodium carbonate:
- in laundry
- for removing permanent hardness of water
- in manufacture of glass, soap, paper, etc
- As a laboratory reagent.
6. PLASTER OF PARIS
Crystalline salts like Na2CO3.10H2O, CaSO4.2H2O, CuSO4.5H2O etc. which seem to be dry contain water of crystallization. Water of crystallization is a fixed number of water molecules present in one formula unit of the salt.
Plaster of Paris(P. O. P) is chemically CaSO4.1/2H2O.
Maufacture
It is prepared by heating gypsum (CaSO4.2H2O) at 100°C
CaSO4.2 H2O → CaSO4.½ H2O + 1½ H2O
Temperature is not allowed rise above 100°C as otherwise is gives anhydrous CaSO4, called "dead burnt plaster" which does not set with water.
PROPERTIES AND USES
It is a white powder. Mixed with water, it sets to a hard mass. This is due to rehydration to form gypsum. It is used for setting fractured bones, in making toys and cast for statues, in making decorative designs on ceilings, pillars, etc., in making chalks and in laboratory to make apparatus air – tight.
Efflorescence
Efflorescence is the property of some substances to lose wholly or partly their water of crystallization when their crystals are exposed to dry air even for a short time. They become powdery. Such substances are called efflorescent substances.
Efflorescent substances lose their water of crystallization and thus their crystalline shape becomes powdery, when exposed to air.
Examples of efflorescent substances:
- Washing soda [hydrated sodium carbonate], when exposed to dry air, becomes a monohydrate.
Na2CO3. 10H2O → Na2CO3.H2O + 9H2O
- Glauber's salt: [hydrated sodium sulphate, Na2SO4. 10 H2O] becomes a powdery anhydrous sodium sulphate when exposed to air.
Na2SO4.10H2O → Na2SO4 + 10H2O
- Epsom salt: [magnesium sulphate heptahydrate], when exposed to dry air, becomes a monohydrate.
MgSO4.7H2O → MgSO4.H2O + 6H2O
The higher the temperature of the air, the higher the efflorescence; this is because air absorbs more water with rising temperature and decreasing moisture.
Hygroscopic Substances
Certain substances absorb moisture (water vapour) from the atmosphere without dissolving in it. Such substances are called hygroscopic substances and the phenomenon is called hygroscopy.
Some examples of hygroscopic substances are:
- Conc. Sulphuric acid H2SO4
- Phosphorus pentoxide (P2O5)
- Quicklime (CaO)
- Silica gel
In the laboratory, these substances are used generally for drying gases, i.e., for removal of moisture form gases.
Deliquescent Substances
Certain water-soluble substances, when exposed to the atmosphere at ordinary temperature, absorb moisture from the atmospheric air to become moist, and ultimately dissolve in the absorbed water, forming a saturated solution. Such a substance is called a deliquescent substance, and the phenomenon is called deliquescence.
Examples:
- Caustic soda (NaOH),
- Caustic potash (KOH),
- Magnesium chloride (MgCl2),
- Zinc chloride (ZnCl2)
- Calcium chloride (CaCl2)
- Ferric chloride (FeCl3)
- Zinc nitrate [Zn(NO3)2],
- Copper nitrate [Cu(NO3)2]
Deliquescence occurs when the vapour pressure of the salt is much lower compared to atmospheric vapour pressure. Thus, de-liquescence is minimized during dry conditions, whereas, efflorescence is maximized in dry conditions.