The Structure of an Atom chapter in Class 9 Science covers the fundamental particles—electrons, protons, and neutrons. With NCERT solutions for Class 9 Science, students understand atomic models by Dalton, Thomson, Rutherford, and Bohr. The class 9 notes provide clear summaries and diagrams for easy recall. Through class 9 science tuition, students receive guidance on atomic numbers, isotopes, and valency with visual aids and practice questions. Our Class 9 tuitions are designed to make chemistry logical and engaging. Understanding atomic structure builds the base for higher-level chemistry studies, making this chapter vital for long-term academic growth.
We have studied earlier that atoms and molecules are the building blocks of matter. The existence of different kinds of matter is due to the presence of different kinds of atoms and molecules in them. Dalton in 1808 suggested that atom is the smallest indivisible particle of matter. He proposed a theory known as Dalton's atomic theory. However, Dalton's concept of atom could not explain the following facts:
- Why do atoms of different elements have different masses and properties?
- Why do atoms of same or different elements combine to form compounds?
A major challenge before the scientists was to reveal the structure of atom as well as to explain its important properties towards the end of nineteenth century. Many experiments were done and it was found that atoms though they are tiny have internal structure and are made up of three main sub-atomic particles namely electrons, protons and neutrons.
Charged Particles In Atom Or Sub-atomic Particles Of Atom
For understanding the nature of charged particles in atoms, let us carry out the following activities:
Activity:
- Comb dry hair. Does the comb then attract small pieces of paper?
- Rub the glass rod with a silk cloth and bring the rod near an inflated balloon. Observe what happens?
Discussion:
- When we comb our dry hair and put the comb near small pieces of paper, we observe that the small pieces of paper get attracted towards the comb.
- When the glass rod rubbed by a silk cloth was brought near the inflated balloon, the inflated balloon got attracted towards the rod.
Conclusion: From the above observation, we conclude that on rubbing two objects together, they become electrically charged. The charge produced shows that atom consists of charged particles also known as sub-atomic particles.
Discovery Of Electron
The existence of electrons in an atom was shown by J.J. Thomson in 1897. He passed electricity at high voltage through a gas at very low pressure taken in a discharge tube.
What is a Discharge Tube?
A discharge tube is a long glass tube closed at both ends. Two circular metal plates A and B are sealed at the two ends of the tube. These circular plates are called electrodes. A side tube S is fused to the tube which can be connected to a vacuum pump (to suck out the air or gas present inside the tube to reduce the pressure inside the tube).
The two plates A and B are connected to a source of electricity to supply high voltage. The plate A connected to the negative terminal is cathode, whereas, the plate B connected to the positive terminal is called anode.
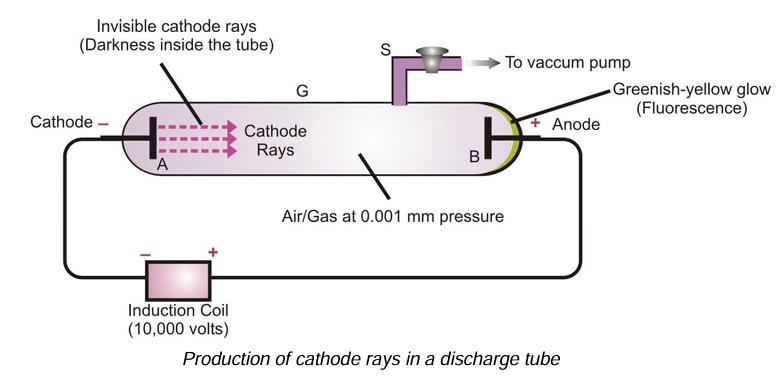
Properties of Cathode Rays
At normal pressure, air or any other gas is a non-conductor of electricity, but at low pressures the gases become conductors of electricity. When sufficiently high voltage is applied across the electrodes, current starts flowing through a stream of particles, moving in the tube from the cathode to the anode. These were called cathode rays or cathode ray particles.
From the various experiments carried by J.J. Thomson and others, the cathode rays have been found to possess the following properties:
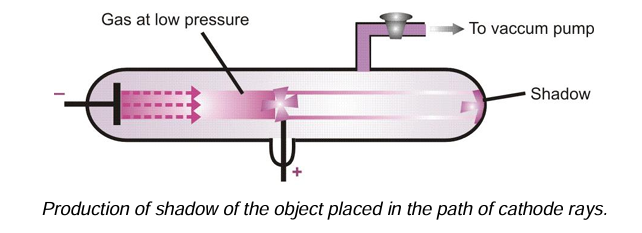
(i) Cathode rays travel in straight lines: This is shown by the fact that if a metal object is placed in the path of the cathode rays, they cast a sharp shadow of the object at the back.
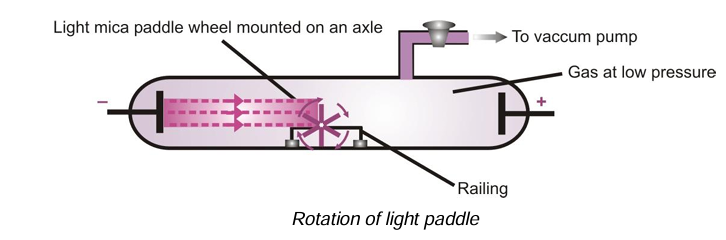
(ii) Cathode rays are made up of material particle: If a large paddle wheel (e.g. that of mica) is placed in their path, the wheel starts rotating. This shows that cathode rays are made up of material particles (particulate nature).
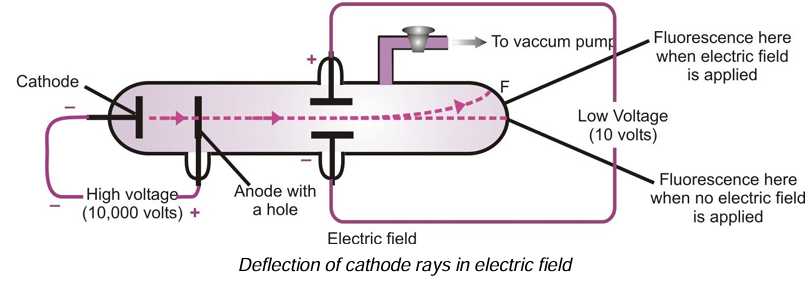
(iii) Cathode rays carry negative charge: When an electric field is applied on the cathode rays, they get deflected towards the positive plate of the electric field. This shows that they carry negative charge.
(iv) When cathode rays strike a metal foil, the foil becomes hot. This indicates that cathode rays produce heating effect.
(v) They cause ionization of the gas through which they pass.
(vi) They produce green fluorescence on the glass walls of the discharge tube as well as on certain other substances such as zinc sulphide (ZnS).
(vii) They produce penetrating effect i.e. they can easily pass through thin foils of metal.
From the study of above properties it was concluded that:
- Cathode rays are made up of material particles.
- Cathode rays carry negative charge.
These negatively charged material particles constituting the cathode rays are called electrons.
Determination of Charge and Mass of Electrons
Further experiments were carried out to determine the exact charge and mass of electrons.
(i) Charge to mass ratio of electron: J.J. Thomson used different discharge tubes fitted with electrodes of different metals. He studied the extent of deflection of cathode rays under influence of electric fields and magnetic fields of different strengths. He placed different gases in the tube. He found that every time the ratio of charge to mass of the electron was the same. This is usually represented by 'e/m', where 'e' represents the charge on the electron and m represents the mass of the electrons. The value was found to be:
Charge/Mass = e/m = 1.76 × 10⁸ C/kg (Coulombs/kg)
(ii) Charge on the electron: Charge on the electron was found by R.A. Millikan. He devised a method known as oil drop experiment to determine the charge on the electrons. He found that the charge on the electron was equal to 1.60 × 10⁻¹⁹ C (1 unit).
This is the smallest quantity of charge that could be measured. Hence, it is also called "one unit charge".
By using the value of e/m and e, the mass of an electron can also be calculated:
m = e/(e/m) = (1.60 × 10⁻¹⁹ C)/(1.76 × 10⁸ C/kg) = 9.1 × 10⁻³¹ kg
Thus, charge on electron is –1 unit and mass is negligible.
Electrons are Constituent of All Atoms
Our studies in the discharge tube experiment conducted by J.J. Thomson have shown that we may take electrodes of any material and we may take any gas inside the discharge tube at low pressure. The cathode ray particles have the same e/m ratio as well as the charge (e) i.e. they carry the same charge and mass. This shows that electrons are constituents of all atoms.
Discovery Of Proton
The existence of positively charged particles in an atom was shown by Goldstein. Electric discharge experiment carried out in the modified cathode ray tube led to the discovery of particles carrying charge. He took a discharge tube with a perforated cathode and a gas at low pressure was taken inside the discharge tube.
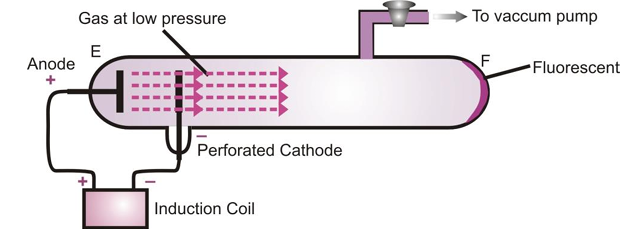
On applying high voltage between the anode and the cathode, it is observed that like cathode rays produce fluorescence on the glass wall of the tube at E, fluorescence is also observed on the glass wall of the tube F. This shows that some rays are also coming from the anode which passed through the holes in the cathode and strike the wall of the tube F. These rays are called anode rays, as they are coming from the side of anode. They are also known as canal rays. Their deflection in an electric field indicates that they carry positive charge.
Properties of Anode Rays / Canal Rays
The characteristic properties of the positively charged rays or anode rays or canal rays are listed below:
(i) They travel in straight lines: If an object is placed in their path, a shadow is produced at the back. This shows that canal rays travel in a straight line.
(ii) They are made up of material particles: Like cathode rays they also rotate the paddle wheel placed in their path. This shows that they are made up of material particles.
(iii) They carry positive charge: In the electric field, these rays are deflected towards the negative plate. It shows that these rays carry positive charge.
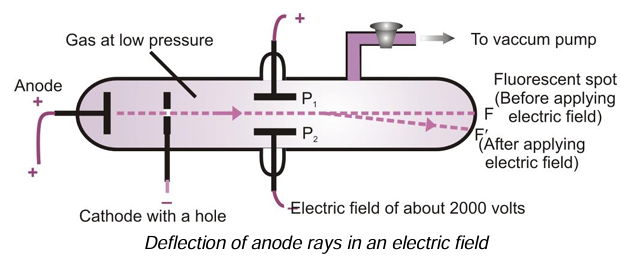
(iv) Determination of charge/mass ratio of the positively charged particles present in anode rays: Unlike cathode rays, the ratio of e/m is found to be different for different gases or we can say that e/m is not constant but depends upon the nature of the gas taken in the discharge tube.
(v) The value of charge on the particles constituting the anode rays is also dependent on the nature of the gas taken inside the discharge tube.
(vi) Mass of the particle constituting the anode rays is also found to be different for the different gases taken in the discharge tube.
Determination of Charge and Mass of Protons
The charge and mass of protons are also determined experimentally like that of electrons.
The charge on these particles is found to be same as that on the electrons, i.e. e = +1.60 × 10⁻¹⁹ C
The ratio of charge/mass (i.e. e/m) = 9.58 × 10⁸ C/kg
The mass of proton m = e/(e/m) = (1.60 × 10⁻¹⁹ C)/(9.58 × 10⁸ C/kg) = 1.67 × 10⁻²⁷ kg
This mass is nearly the same as that of hydrogen atom. When hydrogen gas is used in the discharge tube, the particles constituting the anode rays are called protons. Hence, a proton can be defined as follows:
"That sub-atomic or fundamental particle carrying one unit positive charge and has mass nearly equal to that of hydrogen atom"
As charge on proton is +1 and mass = 1u, hence, it may be represented by the symbol ¹₁P.
Protons are Constituents of All Atoms
If any other gas (other than hydrogen) is taken in the discharge tube, it is observed that the mass of positively charged particle is nearly a whole number multiple of the mass of proton. Hence, it can be concluded that protons are the fundamental particles present in all atoms.
Discovery Of Neutron
In 1932, Chadwick discovered another sub-atomic particle called neutron, by bombarding a thin sheet of beryllium by α-particles. Neutrons are electrically neutral particle i.e. they have no charge and have mass equal to or slightly greater than that of protons. Neutrons are present in the nucleus of all atoms except hydrogen. In general, the neutrons are represented by the symbol 'n'.
A neutron can be defined as the fundamental particle of an atom which has no charge but has a mass nearly equal to that of hydrogen atom.
Comparison of the Characteristics of Electron, Proton and Neutron
| Particle | Charge on the particle | Mass of the particle | Symbol | Location in the atom |
| Electron | –1 unit (–1.602 × 10⁻¹⁹ coulomb) | 9.11 × 10⁻³¹ kg (1/1840 u) | ⁰₋₁e | Outside the nucleus (Extra nuclear part) |
| Proton | +1 unit (+1.602 × 10⁻¹⁹ C) | 1.673 × 10⁻²⁷ kg (1u) | ¹₁p | In the nucleus |
| Neutron | No charge | 1.675 × 10⁻²⁷ kg (1u) | ¹₀n | In the nucleus |
The Structure Of An Atom (atomic Models)
According to Dalton's atomic theory, an atom is indivisible and indestructible. But after the discovery of subatomic particles (electrons, protons and neutrons), various atomic models were proposed by many scientists to explain their arrangement in the atom.
Thomson's Model Of An Atom
To explain the structure of an atom, Thomson proposed a model. According to it, an atom consists of a uniform sphere of positive charge in which electrons are embedded in such a way so as to give the most stable electrostatic arrangement.
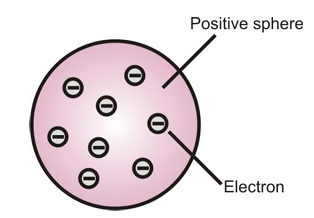
In this model, the atom is visualized as a pudding or watermelon of positive charge with raisins or seeds (electrons) embedded into it.
The important feature of this model is that the mass of an atom is considered to be evenly spread over the atom.
Thomson proposed that:
- An atom consists of positively charged sphere and the electrons are embedded in it.
- The atom as a whole is electrically neutral, showing that negative and positive charges are equal in magnitude.
Limitations: Though it could explain the overall neutrality of the atom, it failed to explain the results of experiments carried out by other scientists.
Rutherford's Model Of An Atom
Earnest Rutherford was interested in knowing how the electrons are arranged within the atom. For this purpose, he performed some experiments known as Rutherford's scattering experiment.
Experiment: In this experiment, he bombarded a thin foil e.g. gold foil (thickness: 100 nm) with a beam of fast moving α-particles. Alpha particles are high energy, positively charged helium ions (emitted during radioactive decay of unstable elements such as uranium) having 2 units of positive charge and 4 units of mass. He observed the scattering of the α-rays after hitting the foil by placing a circular zinc sulphide screen around the metal foil.
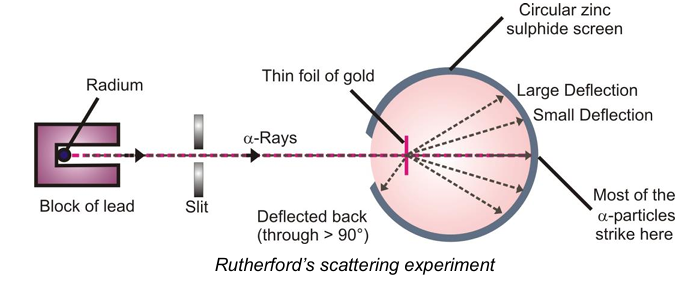
Observations: After the bombardment of α-particles on the thick gold foil, Rutherford observed that:
- Most of the fast moving α-particles passed through the gold foil undeflected.
- Some of the α-particles were deflected by small angles and some were deflected through large angles.
- A very few particles (1 in 20,000) bounced back i.e. were deflected by nearly 180°.
Conclusion: On the basis of these observations, Rutherford drew the following conclusions regarding the structure of atom:
- Most of the space in an atom is empty as most of the α-particles passed through the foil undeflected.
- A few α-particles were deflected from their path. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom, as Thomson had thought. According to Rutherford, the positive charge of the atom occupies very little space. This very small portion of the atom was called nucleus.
- A very small fraction of the α-particles were deflected by 180°, showing that all the positive charge and mass of the gold atom were concentrated in a very small volume within the atom. (Radius of the atom is about 10⁻¹⁰ m while that of nucleus is 10⁻¹⁵ m).
Rutherford's Model
On the basis of above observations, Rutherford proposed the nuclear model of an atom. According to this model:
- An atom consists of a positively charged centre called nucleus.
- The positive charge of the nucleus is due to the protons. On the other hand, the mass of the nucleus is due to the protons and some other neutral particles called neutrons which were discovered later on by Chadwick in 1932.
- The electrons revolve around the nucleus in well defined orbits. Thus, Rutherford's model of atom resembles the solar system in which the nucleus plays the role of sun and the electrons that of revolving planets.
- The atom is electrically neutral because total number of protons in it is exactly equal to the total number of electrons.
- The size of the nucleus is very small as compared to that of an atom.
- Electrons and the nucleus are held together by electrostatic forces of attraction.
To explain that the electrons do not fall into the nucleus as a result of attraction, Rutherford suggested that electrons were not stationary but were moving around the nucleus in certain circular orbits.
Drawbacks of Rutherford's Model of an Atom
Rutherford's model could not explain the stability of an atom. This is because when a particle is moving in a circular orbit, it undergoes acceleration. During acceleration charged particles would radiate energy. Thus, the orbit of the revolving electrons will keep on shrinking or becoming smaller and smaller, following a spiral path and will ultimately fall into the nucleus. However, this actually does not happen and we know that atoms are quite stable.
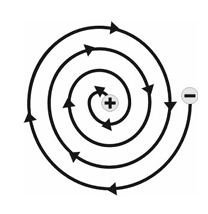
Bohr's Model Of An Atom
In order to overcome the objections raised against Rutherford's model of the atom, Neil Bohr proposed a new model of an atom. To explain the stability of atom, he introduced the concept of the stationary orbital.
Postulates of Bohr's Model
The main points of this Bohr's model of an atom are as follows:
- An atom consists of positively charged nucleus responsible for almost the entire mass of the atom.
- Electrons revolve around the nucleus in certain permitted circular orbits of definite radius and while revolving they do not radiate energy.
- In a particular atom, the orbits in which electrons revolve have fixed radii and energy. These orbits are, therefore called shells or energy levels. These shells are also called stationary states as they have fixed energy. In this manner, Bohr overcame Rutherford's difficulty to account for the stability of the atom.
- The different energy levels were numbered as 1, 2, 3, 4, … etc and called as K, L, M, N … etc. respectively. Greater the distance of energy level from the nucleus, more is the energy associated with it.
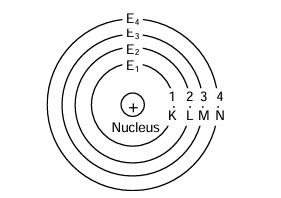
However, the gap decreases between the successive energy shells as we move outwards from the nucleus.
- When electrons move in permitted discrete orbits they do not radiate or lose energy, or gain energy. This stable state of atom is called ground state.
- When energy is given to the electron, it jumps to any higher energy level and said to be in the excited state. In the excited state, the atom is not stable. It tends to lose or emit energy and jumps back to some inner energy level. In other words, when an electron absorbs energy it jumps from inner shell to outer shell whereas when an electron emits energy it jumps from outer shell to inner shell.
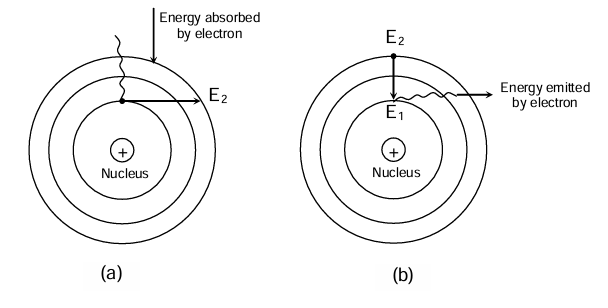
Advantage of Bohr's Model
Bohr's model of an atom explains the stability of an atom by putting the concept of stationary state or energy levels and thus removes the drawback of Rutherford's model of an atom.
How Are Electrons Distributed In Different Orbits (shells)?
As we have discussed earlier that there are a number of shells present in an atom. These are numbered as 1, 2, 3, 4, …, and so on or named as K, L, M, N, … and so on as we move outwards from the nucleus and the electrons are present in these shells.
The distribution (arrangement) of the electrons in the different energy shells of the atom is known as the electronic configuration of that element.
The distribution of electrons in different orbits of an atom was suggested by Bohr and Bury.
Bohr-bury Scheme Of Distribution Of Electrons
The following rules are given by Bohr and Bury for writing the number of electrons in different energy levels or shells:
(i) The maximum number of electrons that can be present in a given shell is equal to 2n², where n = number of shell.
Hence, the maximum number of electrons in different shells can be given as follows:
| Shell | Maximum No. of electrons present |
| 1st shell or K-shell (n = 1) | 2 × (1)² = 2 |
| 2nd shell or L-shell (n = 2) | 2 × (2)² = 8 |
| 3rd shell or M-shell (n = 3) | 2 × (3)² = 18 |
| 4th shell or N-shell (n = 4) | 2 × (4)² = 32 |
(ii) The maximum number of electrons that can be accommodated in the outermost orbit is 8.
(iii) Electrons do not enter into a new shell until and unless the inner shells are completely filled or we can say that shells are filled in a step-wise manner.
Composition of Atoms of the First Eighteen Elements with Electron Distribution
| Element | Symbol | Atomic No. (No. of electrons) | Distribution of electrons in different shells | Short representation of electronic configuration |
| K | L | |||
| Hydrogen | H | 1 | 1 | |
| Helium | He | 2 | 2 | |
| Lithium | Li | 3 | 2 | 1 |
| Beryllium | Be | 4 | 2 | 2 |
| Boron | B | 5 | 2 | 3 |
| Carbon | C | 6 | 2 | 4 |
| Nitrogen | N | 7 | 2 | 5 |
| Oxygen | O | 8 | 2 | 6 |
| Fluorine | F | 9 | 2 | 7 |
| Neon | Ne | 10 | 2 | 8 |
| Sodium | Na | 11 | 2 | 8 |
| Magnesium | Mg | 12 | 2 | 8 |
| Aluminium | Al | 13 | 2 | 8 |
| Silicon | Si | 14 | 2 | 8 |
| Phosphorus | P | 15 | 2 | 8 |
| Sulphur | S | 16 | 2 | 8 |
| Chlorine | Cl | 17 | 2 | 8 |
| Argon | Ar | 18 | 2 | 8 |
| Potassium | K | 19 | 2 | 8 |
| Calcium | Ca | 20 | 2 | 8 |
Image
Valency And Valence Electrons
We have studied earlier about the arrangement of electrons in different shells/orbits.
"The electrons present in the outermost shell/orbit of the atom of an element are called valence electrons."
In all chemical reactions, only the electrons present in outermost orbit will take part in the reaction. As these electrons are present farthest from the nucleus, hence the force of attraction on these electrons by the nucleus is minimum.
According to Bohr-Bury scheme a maximum of 8 electrons can be accommodated in the outermost orbit of an atom. Helium and hydrogen are exception as they can have only two electrons in their outermost shell.
Valency Of An Atom
The concept of valency arises from the study of inert elements. Inert elements are also called noble gases. They have 8 valence electrons (octet) in their outermost orbit/shell or valence shell except helium which has 2 electrons (duplet).
Apart from these elements, all other elements have less than 8 electrons in their valence shell. To attain stability, these atoms lose, gain or share electrons with other atoms to complete their octet. Thus, valency of the atom of an element can be defined as follows:
"The combining capacity of the atoms of an element is known as valency."
Or
"The number of electrons gained, lost or shared by atom of an element in order to complete its octet (or duplet) or to attain stable configuration is known as the valency of the element"
Calculation Of Valency
To calculate the valency of an element, the electronic configuration of the element must be written first and then the valency is calculated. The valency of an element can be calculated as follows:
(i) Elements having 1, 2, 3 and 4 electrons respectively in their valence shell: For these elements valency is equal to the number of electrons present in their valence shell.
(ii) Elements having more than 4 electrons in their valency shell: For these elements having more than 4 electrons in their valence shell, valency can be calculated as follows:
Valency = 8 – Number of valence electrons
Examples: Calculate the valency of fluorine and chlorine elements.
Answer: Fluorine (No. of electrons = 9) and chlorine (No. of electrons = 17) have following electronic configuration:
- Fluorine = 2, 7
- Chlorine = 2, 8, 7
It shows that they have 7 valence electrons. Hence their valency can be calculated as follows:
Valency = 8 – Number of valence electrons = 8 – 7 = 1
Calculate the valency of an element having 5 electrons in their outermost shell.
Answer:Valency = 8 – number of electrons in valence shell = 8 – 5 = 3.
Note: Valency of an anion (M⁻) or cation (M⁺) is equal to the number of charge present on the ion. For e.g. Na⁺, Mg²⁺, Al³⁺ have valency equal to 1, 2 and 3 respectively. Similarly Cl⁻, SO₄²⁻ ions have valency 1 and 2 respectively.
Atomic Number And Mass Number
Atomic Number
As we have studied earlier that protons are present in the nucleus of an atom. It is the number of protons in an atom which determines the atomic number. It is denoted by 'Z'. Thus atomic number can be defined as follows:
"The number of protons present in the nucleus of an atom is known as its atomic number."
So,
Atomic number of an element (Z) = Number of protons in one atom of the element.
Example:
- Nucleus of hydrogen atom contains one proton, its atomic number (Z) = 1.
- Nucleus of carbon atom contains 6 protons, its atomic number (Z) = 6.
Note: All the atoms of the same element have the same number of protons in their nuclei, and hence they have the same atomic number. Two elements can never have the same atomic number, therefore, atomic number can be used to identify an element.
As we know that an atom is electrically neutral, i.e. the number of protons is equal to the number of electrons. Hence, we can say that
Atomic number (Z) = No. of protons = No. of electrons in one neutral atom.
Note: The number of protons or atomic number is equal to the number of electrons only in a neutral atom, and not in an ion.
Mass Number
We already know that the atom of an element contains electrons, protons and neutrons. Since an electron has negligible mass, the mass of the atom is due to the mass of protons and neutrons. Further, as protons and neutrons have mass equal to one atomic mass unit i.e., 1 a.m.u. (1u) on the atomic scale, hence, we can conclude that the mass of an atom is nearly equal to the number of protons and neutrons. This sum is called the mass number of atom and is denoted by 'A'. Thus
Mass number of an element is the sum of protons and neutrons present in the atom of the element. i.e.
Mass number of an element (A) = number of protons + number of neutrons
As both these sub-atomic particles are present in the nucleus of an atom, hence these are also called nucleons. Therefore, we can say that the mass of an atom resides in the nucleus.
In the notation for an atom, the atomic number, mass number and symbol of the element are to be written as:
Mass number / Atomic number Symbol or AZ S
For example:
- Nitrogen is written as = ¹⁴₇N
- Oxygen is written as = ¹⁶₈O
- Sodium is written as = ²³₁₁Na
- Hydrogen is written as = ¹₁H
Note: The mass number of an element is nearly equal to its atomic mass. The main difference is that the mass number is always a whole number while atomic mass can be fractional or not a whole number (as atomic mass is the relative mass as compared with the mass of C-12 atom taken as 12).
Calculation Of Number Of Electrons, Protons And Neutrons, Atomic Number (z) And Mass Number (a)
We know that,
Z = Number of protons (p) = No. of electrons (e) and
A = Number of protons (p) + number of neutrons (n)
But as we know that p = Z
Thus,
A = Z + n
Thus by using above expressions, we can calculate the number of electrons, protons and neutrons in an atom.
Example: If number of electrons in an atom is 8 and number of protons is also 8, then
(i) What is the atomic number of the atom? (ii) What is the charge on the atom?
Answer:
(i) Atomic number (Z) = No. of protons = No. of electrons = 8
(ii) As number of protons and electrons are equal hence the atom will be neutral i.e. there will be no charge on the atom.
Example: Find out the mass number (A) of oxygen and sulphur.
[Given: Number of protons = electrons = neutrons = 8 for oxygen atom and number of protons = neutrons = 16 for sulphur.]
Answer:
(i) Mass number of Sulphur = n + p = 16 + 16 = 32 (ii) Mass number of oxygen = n + p = 8 + 8 = 16
Isotopes
In nature, there are several elements, whose atoms have the same atomic number but different mass number, such atoms are known as isotopes and can be defined as:
"Isotopes are the atoms of the same element, having the same atomic number but different mass number".
As, it is known that
Atomic number (Z) = Number of protons = Number of electrons
So we may conclude that isotopes contain the same number of electrons as well as protons, and we know that
Mass number = Number of protons + Number of neutrons
But as we know that number of protons in them are equal so we can conclude that isotopes of an element differ only in the number of neutrons present in the nucleus.
Example 1: Isotopes of Hydrogen
There are three isotopes of hydrogen, namely protium, deuterium and tritium.
They have only one proton, but differ in the number of neutrons and contain 0, 1, 2 neutrons in their nucleus respectively.
| Isotope | Atomic No. | Mass No. | No. of protons | No. of neutrons | No. of electrons |
| ¹₁H | 1 | 1 | 1 | 1 – 1 = 0 | 1 |
| ²₁H | 1 | 2 | 1 | 2 – 1 = 1 | 1 |
| ³₁H | 1 | 3 | 1 | 3 – 1 = 2 | 1 |
Example 2: Isotopes of Carbon
Carbon has mainly two isotopes which are as follows:
| Isotope | Atomic No. | Mass No. | No. of protons | No. of neutrons | No. of electrons |
| ¹²₆C | 6 | 12 | 6 | 12 – 6 = 6 | 6 |
| ¹⁴₆C | 6 | 14 | 6 | 14 – 6 = 8 | 6 |
Example 3: Isotopes of Chlorine
There are two isotopes of chlorine which are as follows:
| Isotope | Atomic No. | Mass No. | No. of protons | No. of neutrons | No. of electrons |
| ³⁵₁₇Cl | 17 | 35 | 17 | 35 – 17 = 18 | 17 |
| ³⁷₁₇Cl | 17 | 37 | 17 | 37 – 17 = 20 | 17 |
General Characteristics Of Isotopes
(i) Same atomic number: The isotopes of an element have the same atomic number i.e. they have same number of protons and same number of electrons.
(ii) Different mass number: They have different mass number and hence differ in the number of neutrons present in the nucleus.
(iii) Same chemical properties: They have same chemical properties as they have same number of electrons and therefore same electronic configuration and valence electrons.
(iv) Different physical properties: Since they have different mass number hence they differ in their physical properties such as melting point, boiling point, density, etc.
(v) Different nuclear properties: Due to the difference in the number of neutrons in their nucleus they show different nuclear properties e.g. C–14 isotope of carbon is radioactive whereas C–12 isotope is non-radioactive. The radioactive isotope of an element is known as radioisotope.
Fractional Atomic Masses And Calculation Of Average Atomic Masses
The mass of an atom of any natural element is taken as the average mass of all the naturally occurring atoms of that element. Hence, if an element has no isotope then the atomic mass of its atom would be the same as its mass number. But if an element occurs in isotopic form, then we have to know the percentage of each isotopic form to calculate its average atomic mass.
Example: Calculation of average atomic mass can be explained with the help of the following example:
In nature, the two isotopic forms of chlorine viz., ³⁵₁₇Cl and ³⁷₁₇Cl are found in the ratio of 3:1.
Hence,
Average atomic mass = (35 × 75/100) + (37 × 25/100) = (35 × 3/4) + (37 × 1/4) = (105/4) + (37/4) = 142/4 = 35.5 u
This does not mean that any one atom of chlorine has a fractional mass of 35.5 u. It means that if we take a certain amount of chlorine, which consists of both the isotopes of chlorine, then it will have the average atomic mass of 35.5 u. The reason for those fractional atomic masses is that for an element existing in different isotopes, i.e., atoms with different mass numbers, the atomic mass of the elements is the average value which comes out to be fractional.
Applications Of Isotopes
Some isotopes have special properties which find them useful in various fields. Some important and useful applications of the isotopes are given below:
(i) As nuclear fuel: An isotope of uranium (U-235) is used as a fuel in nuclear reactor.
(ii) In medical field:
- An isotope of cobalt (Co-60) is used in the treatment of cancer.
- Phosphorus (P-32) isotope is used in the treatment of leukemia (blood cancer).
- Iodine (I-131) isotope is used in the treatment of goitre.
- Some radio isotopes are used as tracers to detect the presence of tumours, blood clots etc.
(iii) In carbon dating
(iv) In geological dating
Isobars
"Atoms of different elements which have different atomic number but same mass number are called isobars. They have different number of protons, electrons and neutrons but the mass number, i.e. the sum of protons and neutrons in the nucleus is same"
Example: ⁴⁰₁₈Ar, ⁴⁰₂₀Ca
| Isobars | Protons | No. of electrons | No. of neutrons |
| ⁴⁰₁₈Ar | 18 | 18 | 40–18 = 22 |
| ⁴⁰₂₀Ca | 20 | 20 | 40–20 = 20 |
General Characteristics of Isobars
- They are atoms of different elements.
- They have different atomic number.
- They have same mass number.
- They possess different physical as well as chemical properties.
Solved Examples
Question: What are canal rays?
Solution: The positively charged radiations produced in a discharge tube containing a gas at low pressure when a high potential difference is applied between the electrodes, are called canal rays. They are found to travel towards the negatively charged cathode.
Question: If an atom contains one electron and one proton, will it carry any charge or not?
Solution: No, the atom will not carry any charge because electron carries one unit negative charge whereas proton carries one unit positive charge. The net charge on the atom will, therefore, be zero.
Question: On the basis of Rutherford's model of an atom, which sub-atomic particle is present in the nucleus of an atom?
Solution: Proton.
Question: Name the sub-atomic particles of an atom.
Solution: Electron, proton and neutron.