Understanding Natural Resources is an essential part of Class 9 Science. This chapter explains how air, water, soil, and sunlight form the foundation of life on Earth. With NCERT solutions for Class 9 Science, students can explore how these resources are utilized and conserved for future generations. Our class 9 notes simplify concepts like biogeochemical cycles, water conservation, and renewable energy, helping students strengthen their fundamentals. For students looking for personalized help, our class 9 science tuition programs make learning about the environment more interactive and engaging.
Experienced tutors focus on diagrams, examples, and case studies related to natural resource management. Whether you’re preparing for exams or enhancing conceptual clarity, our Class 9 tuitions provide the right academic support. Through structured guidance, students can master important topics like air pollution, water pollution, and soil fertility. With expert tutoring, these environmental concepts become easier to understand and apply in real-world situations.
Introduction: Life on Earth depends on many factors. The energy from the sun and the resources available on the Earth are necessary to meet the basic requirements of all life-forms on the Earth.
Components Of Earth
The outer crust of the Earth is called lithosphere. The water covering 75% of the Earth's surface and also found underground comprise the hydrosphere. The air that forms a blanket around the Earth is called atmosphere.
The life-supporting zone of the Earth where the atmosphere, the hydrosphere and the lithosphere interact and make life possible is known as the biosphere.
Environment
The conditions surrounding an organism and regularly interacting with it forms the environment. It includes both the physical factors and organisms.
Components of Environment:
- Abiotic Components: The physical or non-living factors like air, water, soil, light and temperature
- Biotic Components: The living things like human beings, plants, animals and microbes
Natural Resources
The materials useful to the living organisms and present in the natural environment are called natural resources. The natural resources may be physical (e.g., air, water, minerals, coal) or biological (e.g., microbes, plants, animals).
Types Of Natural Resources
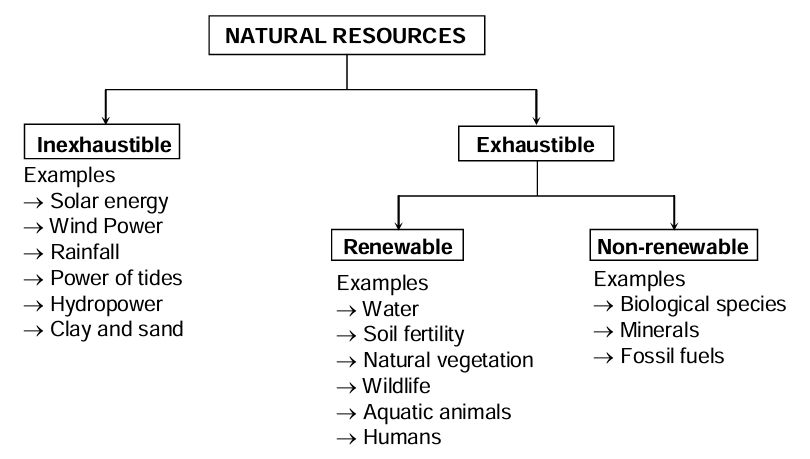
Depending upon its abundance and availability, natural resources are of two types:
Inexhaustible Resources
These are the resources which are unlimited in quantity and are not likely to be exhausted by human use.
Example: solar energy, air, tidal energy, etc.
Exhaustible Resources
These are the resources which are limited and are likely to be finished by human use. They are further of two kinds:
(a) Renewable resources: These resources have the ability to maintain themselves or can be replaced if managed wisely. For example, water, soil, living things like crops, forests, etc. However, they too may be lost by excessive and unwise use.
(b) Non-renewable resources: The non-renewable resources are lost forever as they are not restored. These include metallic minerals, fossil fuels (coal, petroleum), etc.
The Breath Of Life: Air
Air is an inexhaustible natural resource. It consists of mixture of gases such as nitrogen, oxygen, carbondioxide and others. Air also holds water vapours and dust particles.
Composition of Air
| Component | Volume |
|---|---|
| Nitrogen (N₂) | 78.08% |
| Oxygen (O₂) | 20.92% |
| Carbon dioxide (CO₂) | 0.03%** |
| Argon | 0.93% |
| Trace components* | 0.04% |
Interesting Fact: It is interesting to note that even the composition of air is the result of life on Earth. In planets such as venus and mars, no life is known to exist. Carbon dioxide forms the major component constituting upto 95-97% of their atmosphere.
Importance Of Air
Air is essential for the survival and continuity of life as its constituents are needed for various biological processes.
Role of Gases
The various gases present in the air have different functions. They are:
(a) Respiration: The correct proportions of nitrogen and oxygen are required for respiration. In the cell, the oxygen oxidizes sugar molecules to produce carbondioxide and water, and liberates energy. The carbondioxide is expelled out and the energy is used to perform various activities.
(b) Burning: The oxygen of air is essentially required for burning of things like wood, fuels, organic wastes, etc. When they burn in air, carbondioxide and water vapour are produced with the release of heat energy.
(c) Forest fire: It consumes a lot of oxygen.
What is the role of nitrogen in burning?
(d) Photosynthesis: Green plants convert carbondioxide to glucose in the presence of sunlight.
6CO₂ + 12H₂O ────────→ C₆H₁₂O₆ + 6O₂
(e) Formation of shells: Many marine animals use carbonates dissolved in sea water to form their shells.
The Role of Air in Climate Control
Air is a bad conductor of heat. The atmosphere (envelope of air) acts as a protective blanket for living organisms in the following ways:
(a) Air plays a very important role in keeping the temperature of the Earth fairly steady during the day and even during the course of whole year. This happens as a result of a phenomenon known as the green house effect.
(b) Certain gases like carbondioxide, methane, etc. called green house gases prevent the sudden increase in temperature during the day and slows down the escape of heat during the night. The situation on the moon is quite different which is about the same distance from the sun as the Earth. Moon has no atmosphere and the temperature ranges from -190°C to 110°C.
Atmospheric Layers: Atmosphere shows four main concentric layers that differ in density, temperature, composition and properties. The lowest layer close to Earth is troposphere (extends upto 16 kms) and contains moisture, dust particles, gases, etc. Next is stratosphere (upto 16-50 kms) which has a protective ozone shield that protects us from harmful UV radiations. Mesosphere extends upto 50 to 85 kms and beyond this is the thermosphere.
The Movement Of Air: Wind
All the places on Earth are not equally heated up by the sun. When a place becomes heated, the surrounding air also becomes warm. The warm air, being lighter, rises up. A low pressure area is created there. The cold air from the cooler and high pressure regions then moves in to take its place. This movement of air takes place due to uneven heating of different places on the Earth. Due to the unequal heating of places, convection currents are produced in the air. When air moves horizontally, it is called wind.
Water vapours are formed due to the heating of water bodies by solar radiations as well as the activities of living organisms.
The phenomena like movement of air (whether the movement of air will be in the form of a gentle breeze, a strong wind or a terrible storm) and the rain are the result of changes that take place in our atmosphere due to the uneven heating of air and the formation of water vapours. However, couples of other factors like the rotation of Earth, the presence of mountain ranges in the path of the winds also influence.
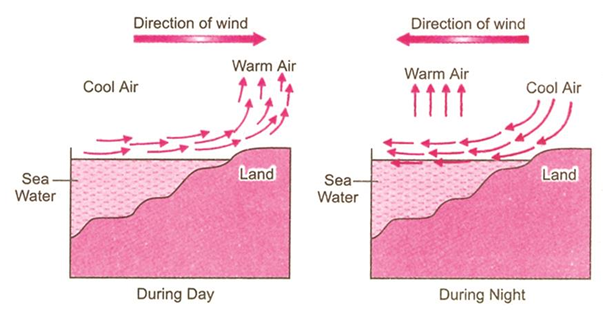
Sea Breeze and Land Breeze
Sea Breeze: During the daytime, the air above the land gets heated faster than water. Hot air above land rises creating a low pressure area and air from the sea moves into this area. Thus, movement of air takes place from the sea side to the land side. This is called sea breeze.
Land Breeze: During night, both the land and the sea start cooling. But the cooling of sea takes place at a slower rate than that of the land. Thus, a low pressure area is created in the sea. The air then moves from the land to the sea. This is called land breeze.
These breezes always occur in the coastal areas.
Clouds and Rain (Condensation and Precipitation of Water Vapour)
Air always contains some amount of water vapour at a given temperature. When the temperature of air falls below a certain temperature, the excess water vapour present in the air gets transformed into tiny droplets of water. This process is called condensation. The temperature at which condensation takes place is called the dew point. A surface called nucleus is required for the condensation to take place. Dust particles, tree leaves, grass, etc. are examples of nuclei.
During the day, a large amount of water vapour goes to air which then rises up, expands and gets cooled. When the temperature falls below the dew point, the excess of water present in the air gets condensed around the dust particles forming tiny droplets of water. When the temperature drops further, these droplets get condensed into tiny crystals of ice which being light floats in the sky. Millions of such droplets come together to form larger droplets called clouds. When these droplets become big enough and heavy, they cannot stay in the air. They come down on Earth as rain.
Fog is the condensation of water vapours at lower layers of atmosphere whereas smog is a combination of smoke and fog in industrial areas. Snow is ice crystals formed by the condensation of water vapour at very low temperature (below 0°C).
Patterns of rainfall of an area or region are determined by the prevailing wind patterns. In large parts of our country, rains are mostly brought by the south-west monsoons and to a smaller extent by north-east monsoons.
Weather reports often say 'depressions' have caused rains in some areas. What are 'depressions'?
Air Pollution
Air pollution is defined as, 'Any undesirable change in the physical, chemical or biological characteristics of the air making it harmful for the living organisms (including man).' Agents or substances that pollute air are called air pollutants.
Sources of Air Pollution
There are two main sources of air pollution:
(a) Natural sources: A number of natural sources do cause air pollution. For instance, volcanic eruptions release toxic gases, ash, heat, etc; forest fires release harmful gases; dust storms put dust particles in the air, etc.
(b) Man-made sources: Man has been polluting the air ever since he started using fire. Urbanization, industrialization, overpopulation, deforestation, burning of fossil fuels, mining activities, etc., all have speeded up the pollution of air.
Harmful Effects of Air Pollution
Air is needed by humans, other land animals and many aquatic organisms for breathing. Its pollution, thus, affect human health. Some of the harmful effects of air pollution are discussed below:
(a) Respiratory problems: The burning of fossil fuels like coal and petroleum produces different oxides of nitrogen and sulphur. These cause bronchitis, asthma, lung cancer, tuberculosis, etc. Similarly, inhalation of polluted air containing dust, cement dust, pollens, etc. may cause sneezing and allergy.
(b) Carbon monoxide poisoning: Carbon monoxide, another toxic air pollutant is emitted from motor vehicles and also cigarette. It affects central nervous system and may lead to 'carbon-dioxide poisoning' in large quantities.
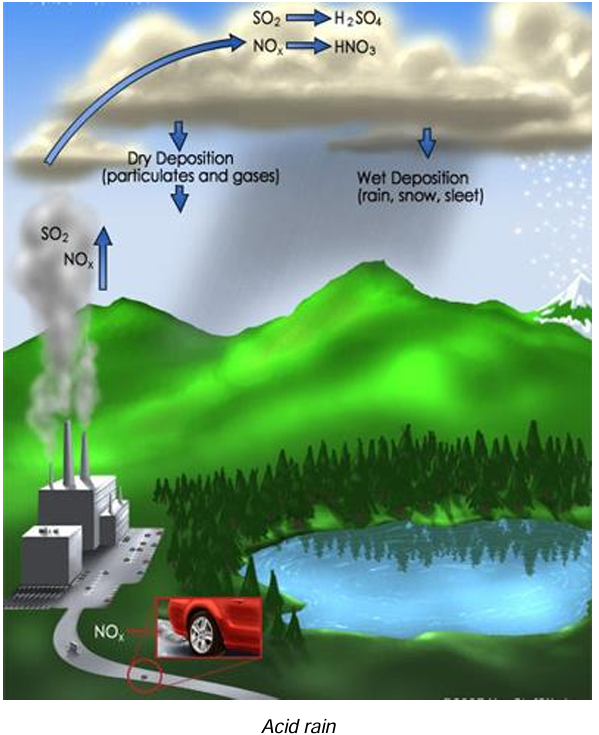
(c) Acid rain: Rain water contains excessive amounts of acids (a mixture of H₂SO₄ and HNO₃ with a pH less than 5). The ratio of two acids depends upon the relative quantities of sulphur dioxide and nitrogen dioxide in the air. Acid rain makes the soil and water in water bodies acidic thereby affecting terrestrial and aquatic life.
(d) Lowering of visibility: The combustion of fossil fuels increases the amount of suspended particles like unburnt carbon particles or hydrocarbons. These pollutants in air cause lowering of visibility, especially in cold weather when water also condenses out of air. This is known as smog and is a visible indication of air pollution.
Lichens as Bio-indicators: Lichens are found to be very sensitive to the levels of contaminants like sulphur dioxide in the air. They die at higher levels of SO₂. Thus, a decrease in lichen population in an area indicates air pollution.
Water: A Wonder Liquid
Water occupies a very large area of the Earth's surface and is also found underground. It is one of the basic necessities of life needed for various activities such as drinking, bathing, washing, etc. It is also needed for irrigation of crops, industries and for navigation. Most of the water on Earth's surface is found in seas and oceans which is saline and constitutes about 97%. Fresh water is found frozen in the ice-caps at the poles and on snow-covered mountains.
The underground water and the water in rivers, lakes and ponds are also fresh. It constitutes about 3%. Availability of water is one of the important factors that decide the sustainability of life in a region. It not only decides the number of individuals of each species that are able to survive in a particular area, but it also decides the diversity of life there. However, the temperature and nature of soil are other factors that also matter.
Availability of fresh water varies from place to place. Practically every summer, most places have to face a shortage of water. And in rural areas, water supply systems have not been installed, so, people are forced to spend considerable amounts of time in fetching water from far-away sources. However, many municipal corporations are trying water harvesting techniques to improve the availability of water.
Rain Water Harvesting
It is a technique used to capture and store rain water by making special water-harvesting structures so that there is an increase in the recharge of underground resources. Dug-out wells, percolation pits, check dams and lagoons can do this job.
Advantages of Rain Water Harvesting
- It reduces the run-off loss of rain water
- It is helpful in controlling floods
- It helps to check soil erosion
- It helps to raise the water table
Necessity Of Water
Water is necessary for the growth and sustenance of both plant and animal lives. Water makes up a large percentage of animal and plant cells.
Importance of Water in Human Life
- Water dissolves all salts and nutrients present in the food
- An aqueous medium is required to carry out all the metabolic reactions
- Water helps in regulating our body temperature
- Water is required for removal of waste products
Importance of Water in Plant Life
- Water helps in germination of seeds and growth of plants
- Green plants require water during photosynthesis
- Water provides a medium for the transport of minerals and food
Water Pollution
An undesirable change in the physical, biological or chemical qualities of water that adversely affects the aquatic life, and makes water less fit or unfit for use, is called water pollution.
Sources of Water Pollution
There are various sources of water pollution. Some of them are discussed below:
(a) Industrial waste: The industrial wastes contain large quantities of harmful chemicals including acids, alkalies and even hot water. For example, mercury salts are used by paper industries.
(b) Fertilizers and pesticides: Fertilizers and pesticides excessively used in the fields to increase crop production are washed by rain water into water bodies and pollute them.
(c) Sewage: Sewage from towns, cities and mostly consisting of organic wastes are dumped into water bodies.
Effects of Water Pollution
(a) Diseases: The pathogens like bacteria, viruses, etc. present in the polluting water cause diseases like typhoid, cholera, jaundice, etc.
(b) Destruction of useful microorganisms: Presence of certain chemicals in industrial wastes kill many useful microorganisms of the water bodies. As these microbes are the natural killing agents of water, therefore self putrification is hindered in these water bodies.
(c) Eutrophication: It is the process by which dissolved oxygen of water is reduced due to excessive growth of algae as a result of extra loading of nutrients in the water body. Dissolved oxygen is used by the animals and plants that live in water. Reduced dissolved oxygen would adversely affect these aquatic organisms.
(d) Imbalance in biodiversity: Hot water released by certain industries or released from dams show marked differences in temperature. This affects the life-forms that are found in these water bodies in many ways. It can encourage the growth of some life-forms and harm some other life-forms. It may affect the breeding as the eggs and larvae of various animals are particularly susceptible to temperature changes.
Mineral Riches In The Soil
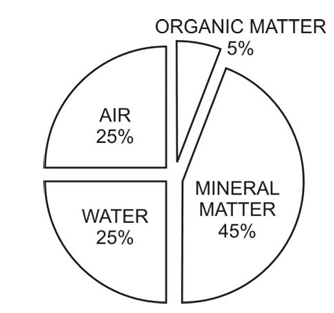
Soil is an important natural resource that decides the diversity of life in an area. It is the top surface layer of the Earth's crust. Rock particles make a large part of the soil. The other constituents which make up the soil are:
- (i) Mineral matter = 45%
- (ii) Organic matter = 5%
- (iii) Water = 25%
- (iv) Air = 25%
- (v) Living organisms
Humus: Humus is a black organic material deposited as a layer over the soil. It is formed by the decomposition of dead plants and animals and supplies nutrients like nitrogen, phosphorus and sulphur to the soil.
Formation Of Soil
The process of soil formation is so slow that the soil is regarded as a non-renewable resource. Over long periods of time, thousands and millions of years, the rocks at or near the surface of the Earth are broken down by various physical, chemical and some biological processes. This is called weathering and results in formation of fine particles of soil.
Physical Weathering
Weathering caused by climatic factors is called physical weathering. The various climatic factors are:
(a) Sun: The rocks get heated up and expand under the influence of solar radiations. Since all the parts of the rocks do not expand and contract at the same time, cracks appear in the rocks and ultimately the large rocks break up into smaller pieces.
(b) Water: Water influences soil formation in the following two ways:
- (i) The water gets into the cracks developed by the unequal heating of the different parts of the rocks. On freezing, the water expands in rock crevices and breaks the rocks.
- (ii) Hard rock wears away over long periods of time by the flowing water. Fast flowing river always carries big and small particles of rock downstream. These rocks rub against other rocks and result in the wearing of rocks into smaller particles. The water takes these particles of rocks and deposits them down its path.
(c) Wind: Strong winds continuously rub against rocks, erode them and thus help in soil formation.
Biological Weathering
Living organisms like lichens, mosses, etc. influence the formation of soil.
(a) Lichens: They live on rocks and produce acids. The acids corrode the surface of rocks to form thin layer of soil.
(b) Mosses: Mosses later grow on such surfaces and cause the rock to break up further.
(c) Roots: The roots of trees enter the cracks and provide anchorage. In due course, roots grow bigger and force the cracks to widen.
Chemical Weathering
The rocks also undergo chemical weathering by processes like hydrolysis, hydration, oxidation and reduction.
Role of Decomposition: Formation of soil also involves decay and decomposition of organic materials (dead remains of plants and animals) by bacteria and fungi resulting in the release of nutrients. Detrivores such as nematodes, earth worms, mites, etc. consume organic matter and add excretory nitrogen to it. Without this process, all the nutrients would remain locked in the dead remains of plants and animals.
Composition Of The Soil
The soil is composed of different types of particles of varying sizes. The various particles are:
- Gravels – Particle size greater than 2 mm in diameter
- Sand particles – Particle size varies from 0.05 mm – 2 mm
- Silt particles – Particle size ranging from 0.005 mm – 0.05 mm
- Clay particles – Particle size less than 0.005 mm
Humus and the micro organisms in the soil decide the quality of the soil. It causes the soil to become porous and allows water and air to penetrate deep underground. The mineral nutrients found in a particular soil depends on the rocks it was formed from.
The nutrient content of a soil, the amount of humus present in it and the depth of the soil are some of the factors that decide which plants will thrive on that soil.
Types Of Soil
On the basis of particle size, soil is classified into the following types:
- Sandy Soil- It consists of large proportion of sand particles and very small quantity of silt and clay. This soil cannot hold much water. It is found in deserts and is unfit for plant growth.
- Clayey Soil- It contains large proportion of clay particles and small amounts of humus and silt. Being compact, it can hold water but cannot trap air. It is also not suitable for plant growth.
- Loamy Soil- It contains relatively larger quantities of clay, silt, sand particles and humus. This is the best soil for plants as it is porous, holding good amount of water and air as well.
Soil (land) Pollution
The contamination of soil with solid waste, chemicals (through industrial wastes or acid rain), fertilizers and pesticides, reducing its fertility is called soil pollution.
Sources of Soil Pollution
The main sources of soil pollution includes:
(a) Solid wastes: The solid wastes coming from residences, cattle sheds, industries, etc. make the surroundings dirty and pollute the soil.
(b) Chemicals: The industrial wastes consisting of a lot of chemicals are generally dumped in vacant sites and they pollute the soil. Some chemicals discharged into the air eventually come down as dry deposition or as acid rain and pollute the soil.
(c) Excess of fertilizers and pesticides: Fertilizers and pesticides when used in excess in agricultural fields pollute the soil. From the soil, many non-biodegradable chemicals (e.g., DDT) even enter the food chain and show biological magnification.
Harmful Effects of Soil Pollution
- Soil pollution reduces the fertility of soil leading to reduction in crop yields
- Solid waste makes the surroundings dirty and also emit foul smell due to their decomposition
- Soil pollution may kill and reduce the diversity of organisms living in the soil including the earthworms, micro-organisms etc. which are instrumental in making the rich humus
Soil Erosion
Soil is a complex mixture of non-living materials and living organisms. It provides anchorage to plants and is also a source of nutrients and water to the plants. Majority of the plants, thus, grow in the soil. The top layer of soil is very fertile and is often carried by environmental agencies.
This removal of the top layer of the soil from one place to another by certain agents such as strong winds, fast flowing water, is called soil erosion.
Causes of Soil Erosion
The various causes of soil erosion are:
(a) Strong winds: Strong winds carry away the loose top soil when it is uncovered.
(b) Heavy rains: Heavy rains or frequent floods wash away unprotected top soil down into the streams, rivers, etc.
(c) Improper farming and suspended cultivation: Farmers loosen the top soil of agricultural fields for cultivation or for removing the weeds. Sometimes, due to certain reasons, when these fields remain uncultivated for a long time it becomes prone to erosion.
(d) Human activities: Large scale deforestation by humans lead to soil erosion.
Effects of Soil Erosion
The various effects of soil erosion are loss of fertility, desertification, landslides, floods, etc.
Prevention of Soil Erosion
Certain measures can effectively reduce soil erosion. These include intensive cropping, sowing grasses in uncultivated land, terrace farming along the slopes of hilly areas, making embankments along the river banks, etc.
Biogeochemical Cycles
Both non-living (abiotic) and living (biotic) components of the biosphere constantly interact with each other to form a dynamic, but stable system. The interactions include transfer of matter and energy between the different components of the biosphere.
The nutrient elements derived from the Earth by the living organisms for use in their growth and metabolism are called biogeochemicals. These are continuously recycled.
The movement of nutrient elements through the living and non-living components of the biosphere or any other ecosystem, is called biogeochemical cycle or cycle of matter.
Nutrient Cycling: Soil is the reservoir of nutrients. The process of transfer of nutrients from the soil through absorption to the plants is termed as uptake. When the uptake of nutrients is more than the amount of nutrients recycled (e.g. in a young growing forest), a part of the uptake is retained in the standing crop which increase the nutrient content of the ecosystem. Therefore, Retention = Uptake – Recycle.
The Water-cycle Or Hydrological Cycle
Water is constantly exchanged between the air, land and sea, and between the living organisms and their environments. There are two overlapping water cycles in nature.
Global Water Cycle
Water evaporates from the hydrosphere (oceans, seas, rivers, lakes, etc.) with sun's heat and form clouds. Rain may fall directly into the oceans or clouds blowing over the land also precipitate (rain, snow, hail, etc). Some water is soaked into the ground and some flows into rivers from where it gets into the seas. The ground water comes to the surface by springs and pumps. This water finally evaporates into the atmosphere and form clouds. This completes the global water cycle.
Biological Water Cycle
Living organisms also cycle water. For example, animals drink water and throw out water as urine and sweat. Plants absorb water from the soil and lose water to the atmosphere through transpiration. Water is also added to the environment by death and decay of organisms. This water form clouds and enter the global cycle.
Water is used by terrestrial plants and animals for various life processes. Transpiration of water plays a role in determining the microclimate around them. Water being a universal solvent dissolves a large number of substances. These dissolved minerals are carried to rivers and then to the seas where these are used by the marine animals.
Nitrogen Cycle
Nitrogen comprises about 78% of the Earth's atmosphere. It is an important constituent of plant and animal protein, which forms the structural and functional component of cells. Nitrogen is also present in DNA, RNA, vitamins, urea and alkaloids. Nitrogen is thus, an essential nutrient for all life-forms and life would be simple if all these life-forms could use atmospheric nitrogen directly.
The cyclic pathway by which nitrogen is circulated continuously through the living and non-living components of the biosphere is called the nitrogen cycle.
The nitrogen cycle can be studied under the following heads:
Nitrogen Fixation
The process of converting free nitrogen of the atmosphere into compounds of nitrogen is called nitrogen fixation.
Nitrogen cannot be directly absorbed by plants and animals other than a few forms of bacteria. They can take up nitrogen in the form of nitrites and nitrates. By nitrogen fixation, the atmospheric nitrogen enters the living system. This can occur in three ways:
(a) Atmospheric fixation: The high temperatures and pressures produced during lightening allow nitrogen to combine with oxygen in the atmosphere to form oxides of nitrogen. These dissolve in rainwater and get soaked into the soil to form nitrates.
N₂ + O₂ → 2NO
2NO + O₂ → 2NO₂
4NO₂ + O₂ + 2H₂O → 4HNO₃
Nitric acid is carried to the soil by rain water where it forms nitrates. These nitrates being soluble in water are then absorbed by plants.
(b) Biological fixation: Root nodules of certain leguminous plants like peas and beans have nitrogen-fixing bacteria (Rhizobium) which convert atmospheric nitrogen to nitrogen compounds.
(c) Industrial fixation: In this method, the nitrogen of the atmosphere is made to combine with hydrogen to form ammonia (NH₃). Ammonia is either oxidized to form nitrates or made to react with acids to form ammonium salts. These nitrates and ammonium salts are used as fertilizers.
Ammonification
The process of conversion of complex organic compounds (plants and animals proteins) into ammonia is called ammonification.
Some bacteria present in the soil convert the proteins of dead plants and animals into ammonium ions (NH₄⁺). A number of animals excrete urine containing urea which is converted into ammonia by the process of ammonification. Some types of bacteria synthesize proteins from ammonia.
Nitrification
It is the process of conversion of ammonia into nitrites and nitrates. Ammonia is acted upon by nitrifying bacteria (Nitrosomonas & Nitrobacter) and is changed to nitrates.
NH₃ + O₂ → NO₂⁻ + H₂O + Energy
NO₂⁻ + O₂ → NO₃⁻ + Energy
The nitrates formed in the second step are available for recycling.
Denitrification
It is the process of conversion of nitrate salts to free nitrogen. It is carried out by free-living denitrifying bacteria (Pseudomonas) present in the soil.
Thus, there occurs a nitrogen cycle in nature by which nitrogen passes from its elemental form in the atmosphere into simple molecules in the soil and water. These, then get converted into complex molecules in living beings and back again to the simple nitrogen molecules in the atmosphere.
Carbon Cycle
Carbon is the basic constituent of all living beings. Its organic compounds play a major role in our life as carbohydrates, fats, proteins and nucleic acids. Food, fibers (cotton, jute), medicines, fertilizers and fuels, all contain compounds of carbon. The endoskeletons and exoskeletons of various animals are also formed from carbonate salts.
There are three main reservoirs of carbon:
- Atmosphere, in the form of carbon dioxide
- Oceans in the form of dissolved CO₂
- In carbonate and hydrogen carbonate salts in various minerals, coal, graphite, diamond, petroleum, etc.
Carbon is incorporated into life forms by the process of photosynthesis in the presence of sunlight and chlorophyll. This process converts carbon dioxide from the atmosphere or dissolved in water into glucose molecules. These glucose molecules are either converted into other complex substances or used to provide energy for the synthesis of other biologically important molecules. The carbon from plants passes through different living beings as food.
Carbon dioxide returns to the atmosphere through respiration in plant and animals, decomposition of dead plants and animals, volcanic eruptions and combustion of fossil fuels.
Thus, a carbon cycle operates in our environment, as a result of which the proportion of carbon dioxide in the atmosphere remains almost the same.
Oxygen Cycle
Oxygen is a very abundant element in our Earth. It is found in the elemental form in the atmosphere (21%). In combined form, it is found in the Earth's crust as oxides of most metals and silicon, as carbonates, sulphates, nitrates and other minerals. It is also found in the air in the form of carbon dioxide. Oxygen is also an essential component of most biological molecules like carbohydrates, proteins, nucleic acids, fats, etc.
All living organisms take in oxygen for respiration where food is oxidized, energy is produced and carbon dioxide is released into the atmosphere.
This carbon dioxide is used by green plants during photosynthesis and oxygen is released, some of the oxygen also gets incorporated into the food material (carbohydrates).
Oxygen is also cycled during burning and combustion. Oxygen is consumed in the burning of materials and carbon dioxide is released during this process. Oxygen combines with nitrogen and forms nitrogen oxides, amino acids, proteins, etc. These compounds release oxygen in the atmosphere when they are broken down. Thus, oxygen level is balanced in the environment.
Global Warming (green House Effect)
A greenhouse is a glass building used for growing plants that need protection from cold. Glass reflects infrared radiations of longer wavelengths but allows those with shorter wavelengths to pass through. This behaviour of glass is used to make a greenhouse. The solar radiation which passes through the glass heats up the surfaces inside the greenhouse.
These warm surfaces then radiate heat. These radiations consist mainly of IR (infra-red radiations) of longer wavelengths. They are reflected by the glass of the greenhouse. Thus, the heat inside the green house is trapped which keeps the green house warm.
A similar effect takes place in the atmosphere. Gases like carbon dioxide (CO₂), methane (CH₄), nitrous oxide, etc. are called green house gases. Out of these CO₂ is the most important green house gas. The CO₂ behaves like a glass and allows IR radiations of shorter wavelengths to pass through, but reflects the IR radiations of longer wavelengths. During the day, solar radiations including IR radiations of shorter wavelengths, heat up the surface of the Earth. The surface of the Earth then reradiates some of these radiations in the form of heat. The IR radiations from the surface of the Earth have longer wavelengths. So, they are reflected by the carbon dioxide in the atmosphere. The heat is thus trapped which keeps the Earth warm even during the night.
Man is adding large amounts of CO₂ and methane to the atmosphere by burning of fuels and by deforestation, etc. These gases heat up the atmosphere more than normal leading to an increase in Earth's temperature. This phenomenon is called global warming which occurs due to green house effect.
Ozone Layer
Elemental oxygen is normally found in the form of a diatomic molecule. However, in the upper reaches of the atmosphere a molecule containing three atoms of oxygen (O₃) called ozone is formed under the influence of UV radiations (ultraviolet radiations). This ozone exists as thick blanket called ozone shield in the stratosphere.
UV radiations
O₃ ⇌ O₂ + [O]
Ozone Oxygen Nascent oxygen
Ozone shield performs a useful function. It absorbs harmful UV radiations of the sun and prevents them from reaching the surface of the Earth otherwise they may damage many forms of life.
Depletion Of Ozone Layer
It was discovered recently that the ozone layer was getting depleted. A class of compounds of carbon, fluorine and chlorine called chlorofluorocarbons (very stable compound not degraded by any biological process) are freely used in air-conditioners, refrigerators, aerosol sprays, etc. These compounds rise up very high in the atmosphere and break down to form chlorine atoms which interact with ozone to form oxygen.
CFCl₃ ──light──→ CFCl₂ + Cl
Chlorofluorocarbon Chlorine
Cl + O₃ → ClO + O₂
Chlorine monoxide
ClO + O → Cl + O₂
As ozone gets converted into oxygen, the amount of ozone in the ozone layer also decreases. Over the time, this has created an ozone-deficient area in the atmosphere. This area is called the ozone hole. This is most prominent over Antarctica.
Effects Of The Ozone Hole
- Ultra violet radiations have many harmful effects. Without the shield of ozone layer, UV rays may directly come on the Earth causing skin cancer, cataract, etc.
- Ultraviolet rays adversely affect plant life too.
Solved Examples
Question: What is the major source of fresh water in the city/town/village where you live?
Solution: Major source of fresh water in the city/town/village where we live is underground water.
Question: Define natural resources.
Solution: The materials present in natural environment (atmosphere, lithosphere and hydrosphere) are useful to life are called natural resources.
Question: Name two common pathogens in polluted water.
Solution: Bacteria and protozoa
Question: Name the two important biological processes in which air is essential.
Solution: Respiration and photosynthesis
Question: Give two effects of soil pollution.
Solution:
- (i) Reduction of fertility of soil
- (ii) Desertification
Question: What is rain-water harvesting?
Solution: The technique used to capture and store rain water by making special water-harvesting structures so that there is an increase in the recharge of underground water resources.
Question: What are the methods of preventing or reducing soil erosion?
Solution: Soil erosion can be effectively prevented by –
- (i) Intensive cropping
- (ii) Sowing grasses and planting xerophytes
- (iii) Terrace farming
- (iv) Proper drainage canals around the fields, and
- (v) Making strong embankments along the river banks
Question: You have seen weather reports on television and in newspapers. How do you think we are able to predict the weather?
Solution: We daily see weather reports on televisions and newspapers. This information is actually recorded by meteorological laboratories present in different cities of the country. Information such as direction and speed of wind, average daily minimum and maximum temperature, relative humidity, patterns of cloud formation, depression zone over an area, etc., are recorded with the help of instruments and then displayed on television or published in newspapers. This meteorological information helps us to predict the weather.
Question: Write a note on how forests influence the quality of our air soil and water resources.
Solution: Forests influence the quality of our air, soil and water resources in the following ways:
- (i) Plants maintain the CO₂ and O₂ balance in the atmosphere.
- (ii) Roots of plants bind the soil and do not allow erosion of soil by fast winds or fast moving water. In this way, they help in maintaining the fertility of the soil. Many bacteria present in root nodules (e.g., Rhizobium bacteria in root nodules of leguminous plants) replenish nitrogen to the soil.
- (iii) By preventing soil erosion, forests maintain the quality of water-resources as well as reducing silting.