Alcohols
Alcohols are the class of organic compounds containing one or more hydroxyl groups attached directly to carbon atoms. Alcohols may thus be regarded as derivatives of water in which one hydrogen atom has been replaced by an alkyl group.
Compounds with one –OH group are called monohydric alcohols; those with more than one are di-, tri- and polyhydric alcohols.
General formula of monohydric alcohols: CnH2n+1-OH
Types of Alcohols
Monohydric Alcohols
- Primary (1°): CH₃CH₂OH
- Secondary (2°): CH₃-CH(OH)-CH₃
- Tertiary (3°): (CH₃)₃C-OH
Dihydric Alcohol
Ethylene glycol: H₂C(OH)-CH₂(OH)
Trihydric Alcohol
Glycerol: H₂C(OH)-CH(OH)-CH₂(OH)
Polyhydric Alcohol
Sorbitol: H₂C(OH)-CH(OH)-CH(OH)-CH(OH)-CH(OH)-CH₂(OH)
Note:Generally, polyhydric alcohols are derived from sugars.
Methods of Preparation of Alcohol
From Alkene
(i) Hydration Method:
R-CH=CH₂ + H₂O → R-CH(OH)-CH₃
Conditions: dil. H₂SO₄
(ii) Hydroboration-Oxidation Method:
R-CH=CH₂ + BH₃ → R-CH₂-CH₂-B → R-CH₂-CH₂-OH
Conditions: OH⁻/H₂O₂
(iii) Oxymercuration-Demercuration Method:
R-CH=CH₂ + Hg(OCOCH₃)₂/H₂O, 25°C → R-CH(OH)-CH₃
Followed by NaBH₄ treatment
From Alkyne
R-C≡CH + H₂SO₄/H₂O/Hg²⁺ → R-CO-CH₃ Rearrangement occurs
From Alkyl Halide
1°, 2° and 3° alkyl halides are hydrolyzed by aqueous alkalies or moist silver oxide to give 1°, 2° and 3° alcohols respectively.
R-X + HOH/NaOH → R-OH R-X + Moist Ag₂O/AgOH → R-OH
Examples:
(a) CH₃CH₂Cl + Moist Ag₂O → CH₃CH₂OH + AgCl
(b) (CH₃)₃C-Cl + Moist Ag₂O → (CH₃)₃C-OH
Note: Ag₂O + H₂O → 2AgOH
Reduction Methods
(i) Reduction of Carbonyl Compounds:
(a) Aldehyde to 1° alcohol: R-CHO + H₂/Ni → R-CH₂OH
(b) Ketone to 2° alcohol: R-CO-R' + H₂/Ni → R-CH(OH)-R'
Drawback of H₂/Ni reduction: Double bonds and triple bonds are also reduced.
Example: CH₃-CH=CH-CHO + H₂/Ni → CH₃-CH₂-CH₂-CH₂OH
Cannot prepare: CH₃-CH=CH-CH₂OH
Reduction by LiAlH₄ or NaBH₄:
Aldehydes, ketones, esters, amides and halides are reduced by LiAlH₄ to alcohols. Aldehydes and ketones are also reduced by NaBH₄.
R-CHO + LiAlH₄/H₃O⁺ or NaBH₄ → R-CH₂OH
R-CO-R' + LiAlH₄/H₃O⁺ or NaBH₄ → R-CH(OH)-R'
From Grignard Reagent
(i) Formation of Primary Alcohol:
Grignard reagent reacts with formaldehyde and epoxy ethane to form primary alcohol.
(a) H₂C=O + CH₃MgI → CH₃-CH₂-OH + Mg(OH)I
(b) H₂C-CH₂ (epoxide) + CH₃MgI → CH₃-CH₂-CH₂-OH
(ii) Formation of Secondary Alcohol:
Grignard reagent reacts with acetaldehyde and ethyl formate to give secondary alcohol.
(a) CH₃CHO + CH₃MgI → CH₃-CH(OH)-CH₃
(b) H-COOEt + CH₃MgI (excess) → CH₃-CH(OH)-CH₃
(iii) Formation of Tertiary Alcohol:
(CH₃)₂C=O + CH₃MgI → (CH₃)₃C-OH
Chemical Properties of Alcohol
Alcohols are reactive compounds mainly due to the presence of hydroxyl group. The reactions can be broadly divided into three categories, namely:
- Reactions involving the cleavage of C–OH bond
- Reactions involving the cleavage of O–H bond
- Reactions involving the cleavage of both alkyl and hydroxyl group
Reactions Involving the Cleavage of C–OH Bond
(i) Reaction with PCl₅, PCl₃ and SOCl₂:
(a) R-OH + PCl₅ → R-Cl + POCl₃ + HCl
(b) 3R-OH + PCl₃ → 3R-Cl + H₃PO₃
(c) R-OH + SOCl₂ (Pyridine) → R-Cl + SO₂ + HCl
[Note: HCl is dissolved in pyridine and SO₂ escapes out. Hence, pure alkyl halide is formed.]
(ii) Reaction with Ammonia:
R-OH + NH₃ (Al₂O₃, 360°C) → R-NH₂ (ROH, Al₂O₃) → R₂NH (ROH, Al₂O₃) → R₃N
Reactions Involving the Cleavage of O–H Bond
(i) Reaction with Metals: Alcohol reacts with metal to form alkoxide and hydrogen gas is released.
R-OH + Na → R-O⁻Na⁺ + ½H₂ 2R-OH + Ca → (R-O)₂Ca + H₂
(ii) Reaction with Organic Acids:
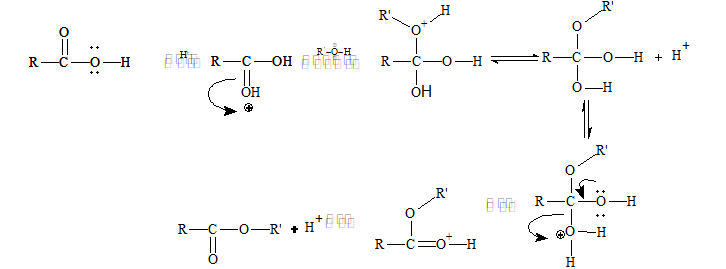
Alcohol reacts with organic acids to form esters. The process is known as esterification.
R-COOH + HO-R' (conc. H₂SO₄) → R-COO-R' + H₂O
Mechanism of Acid Catalysed Esterification:
(iii) Reaction with Inorganic Acids: Alcohols react with inorganic acids to form inorganic esters.
R-OH + HO-NO₂ → R-O-NO₂ + H₂O (Alkyl nitrate)
Exercise 1:
Action of HNO₂ on CH₃NH₂ gives:
- CH₃OH
- CH₃OCH₃
- CH₃–O–N=O
- Both (B) and (C)
(iv) Alkylation:
When alcohols react with dialkyl sulphates, the hydrogen of the hydroxyl group is replaced by an alkyl group resulting in the formation of ether.
C₂H₅OH + (CH₃)₂SO₄ → C₂H₅OCH₃ + CH₃HSO₄ (Ether)
Reactions Involving the Cleavage of both Alkyl and Hydroxyl Group
(i) Oxidation:
The oxidation of alcohol depends upon the nature of alcohol. The oxidizing agents usually employed are: acidified potassium dichromate (K₂Cr₂O₇), acidified or alkaline potassium permanganate or dilute nitric acid.
Oxidation of Primary Alcohol: A primary alcohol is easily oxidized to an aldehyde and then to carboxylic acid.
R-CH₂OH → R-CHO → R-COOH (mild oxidizing agents)
Oxidation of Secondary Alcohol: On oxidation, secondary alcohol forms ketone.
R-CH(OH)-R' → R-CO-R' (mild oxidizing agents)
Note: Besides above mentioned oxidizing agents, few other mild oxidizing agents are also used, viz., X₂, Fenton's reagent [FeSO₄/H₂O₂], acidic K₂Cr₂O₇, Jones reagent (CrO₃/PCC), Cu/300°C.
Tertiary Alcohols
Give ketone of less number of carbon atoms:
(CH₃)₃C-OH + acidic K₂Cr₂O₇ → (CH₃)₂C=O + CO₂ + H₂O
Give alkene with Cu/300°C:
(CH₃)₃C-OH + Cu/300°C → (CH₃)₂C=CH₂ + H₂O (dehydration)
(ii) Dehydration:
Alcohols on dehydration (elimination of water molecule) forms alkene. Dehydration takes place easily in case of tertiary alcohol in comparison to primary alcohol.
(a) CH₃CH₂OH → CH₂=CH₂ Conditions: (i) Al₂O₃ at 350°C or (ii) conc. H₂SO₄ at 170°C
(b) CH₃CH₂C(CH₃)(OH)H → CH₃CH=C(CH₃)₂ Conditions: (i) Al₂O₃ at 250°C or (ii) H₂SO₄
Specific Reaction
Pinacol–Pinacolone Rearrangement
Pinacols on treatment with mineral acids loses a molecule of water and rearranges to form pinacolone.
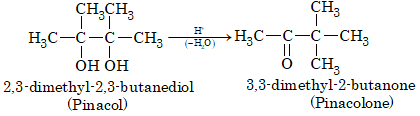
Ethers
A class of organic compounds which contain C–O–C linkage, i.e. these are derivatives of water (H–O–H) where both the hydrogen atoms are replaced by alkyl or aryl groups. Ethers which contain two identical alkyl or aryl groups are called symmetrical ethers, and those containing two different groups are termed as unsymmetrical ethers.
Methods of Preparation of Ethers
From Alcohols
(i) Dehydration Method:
Primary alcohols on dehydration with conc. H₂SO₄ at lower temperature form ether.
2R-OH + conc. H₂SO₄ (140°C) → R-O-R + H₂O
Note: Lower temperature favours the formation of ether whereas higher temperature favours the formation of alkene.
(ii) Reaction with Diazomethane:
Alcohol reacts with diazomethane to give alkyl methyl ether.
R-OH + (CH₂N₂)Al₂O₃ → R-OCH₃ + CH₂=N₂
From Sodium Alkoxide (Williamson Synthesis)
A nucleophilic substitution reaction between sodium alkoxide and alkyl halide gives ether. It is known as Williamson synthesis.
R-ONa + R'-Cl (SN2) → R-O-R' + NaCl
Note:
(i) Alkyl halide should be primary because secondary and tertiary alkyl halides undergo dehydrohalogenation in the presence of R-O⁻, which is a strong base and follows elimination reaction.
CH₃CH₂CH₂ONa + CH₃CH₂Br → CH₃CH₂CH₂-O-CH₂CH₃
(ii) Vinyl halide and aryl halides are not used due to their low reactivity.
Physical Properties of Ethers
- Lower members are gases or volatile liquids: CH₃OCH₃ and CH₃OC₂H₅ (gases)
CH₃CH₂OCH₂CH₃ (volatile liquid) - Appearance: They are colourless liquids with pleasant ethereal odour.
- Inflammability: Ether vapours are highly inflammable. Ether fire is extinguished by CO₂ or pyrene (CCl₄) extinguisher and not by water as ether floats on water.
- Dipole nature: The C–O bond in ether is polar in nature as oxygen is more electronegative than carbon atom. They have a dipole moment of 1.15-1.30 D. The two C–O bonds are inclined at an angle of 110°, hence two dipoles do not cancel each other and makes ether somewhat polar.
- Boiling points: Ethers are isomeric with alcohols, but their boiling points are much lower than those of the isomeric alcohols as ethers do not form intermolecular hydrogen bonding.
- Solubility: Ethers containing upto three carbon atoms are soluble in water due to the formation of hydrogen bonds between water and ether molecules.
Exercise 2:
Which of the following exhibits maximum hydrogen bonding?
- C₂H₅OH
- C₂H₅OC₂H₅
- C₂H₅Cl
- Et₃CNH₂
Chemical Reactions of Ethers
(i) Nucleophilic Substitution Reactions:
Ether reacts differently with cold and hot HI.
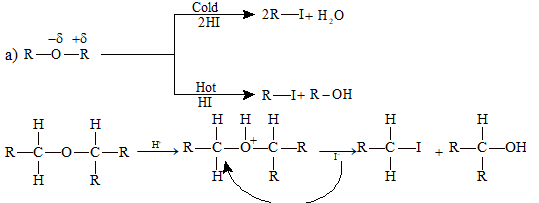
Excess reagent reaction: If the reaction is carried out in presence of excess of reagent, the byproduct alcohol (formed) gets converted into the corresponding halide.
R-O-R' + 2HI (excess) → R-I + R'-I + H₂O
Note: Reaction of ether with HI is used for the estimation of alkoxy groups and the method is known as 'Zeisel' method.
(ii) Halogenation:
(a) α-H atoms are displaced by Cl atoms, when halogenation takes place in dark.
CH₃CH₂-O-CH₂CH₃ + Cl₂ (Dark) → CH₃CHCl-O-CHClCH₃
(b) When halogenation takes place in presence of light and excess of halogen, all the H atoms are displaced by Cl atoms.
CH₃CH₂-O-CH₂CH₃ + Cl₂ (excess, hν) → CCl₃CCl₂-O-CCl₂CCl₃ (perchlorodiethylether)
Phenols
The class of organic compounds in which hydroxyl group is directly linked to the benzene ring. Similarly, like alcohol phenols can be monohydric, dihydric and trihydric.
Methods of Preparation of Phenol
From Benzene Sulphonic Acid:
Benzensulphonic acid on fusion with NaOH gives sodium salt of phenols.
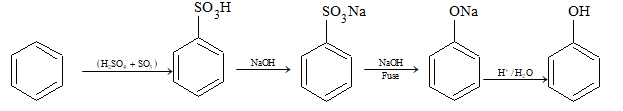
From Chlorobenzene (Dow's Process):
This process involves alkaline hydrolysis of chlorobenzene.
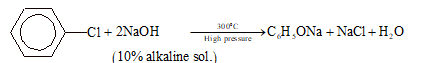
2C₆H₅ONa + H₂SO₄ → 2C₆H₅OH + Na₂SO₄
Rasching's Process:
C₆H₆ + HCl + ½O₂ (CuCl₂/FeCl₃, 250°C) → C₆H₅Cl + H₂O
C₆H₅Cl + H₂O (425°C) → C₆H₅OH + HCl
From Benzene Diazonium Chloride:
C₆H₅N₂⁺Cl⁻ + H₂O (warm) → C₆H₅OH + N₂ + HCl
From Grignard Reagent:
C₆H₅Br + Mg (Ether) → C₆H₅MgBr + O₂ → C₆H₅OMgBr + H⁺/H₂O → C₆H₅OH
Oxidation of Benzene:
This is the latest method for the manufacture of phenol.
2C₆H₆ + O₂ (V₂O₅, 315°C) → 2C₆H₅OH
Oxidation of Isopropyl Benzene (Cumene):
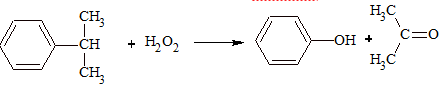
Physical Properties
Phenol is a colourless, crystalline and deliquescent solid. It attains pink colour on exposure to air and light. It has a peculiar characteristic smell and a strong corrosive action on skin.
Chemical Properties
Acidic Nature of Phenol
Phenol is a weak acid. The acidic nature of phenol is due to the formation of stable phenoxide ion in solution.
C₆H₅OH + H₂O ⇌ C₆H₅O⁻ + H₃O⁺
The phenoxide ion is stable due to resonance:
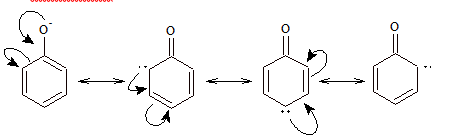
The negative charge is spread throughout the benzene ring. This charge delocalization is a stabilizing factor in the phenoxide ion and increase acidity of phenol.
Reactions of –OH Group
(i) Reaction with FeCl₃:
Phenol gives violet colouration with FeCl₃ solution due to the formation of a coloured ion complex, which is a characteristic to the existence of keto-enol tautomerism.
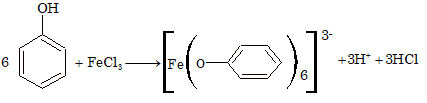
(ii) Ether Formation:
C₆H₅OH + NaOH → C₆H₅ONa + H₂O
C₆H₅ONa + ClCH₃ → C₆H₅OCH₃ + NaCl (Anisole)
C₆H₅ONa + ClCH₂CH₃ → C₆H₅OC₂H₅ + NaCl (Phenetol)
(iii) Ester Formation (Schotten–Baumann reaction):
(a) C₆H₅OH + NaOH → C₆H₅ONa + H₂O
C₆H₅ONa + ClCOCH₃ → C₆H₅OCOCH₃ + NaCl
(b) C₆H₅OH + (CH₃CO)₂O (NaOH) → C₆H₅OCOCH₃ + CH₃COOH
Note: The phenyl esters on treatment with anhydrous AlCl₃ undergoes Fries rearrangement to give o–and p–hydroxy ketones.
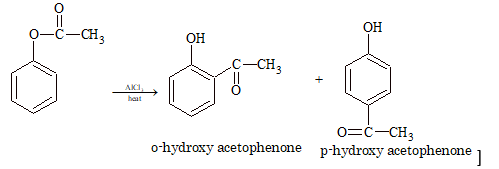
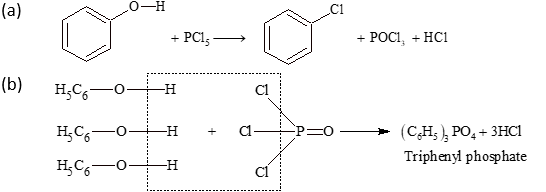
(iv) Reaction with PCl₅:
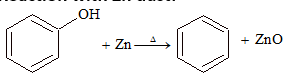
(v) Reaction with Zn dust:
(vi) Reaction with NH₃ (Bucherer reaction):
C₆H₅OH + H₂NH₂ (ZnCl₂, 300°C) → C₆H₅NH₂ + H₂O (Aniline)
Reactions of Benzene Nucleus
The –OH group is ortho and para directing. It activates the benzene nucleus.
(i) Halogenation (Bromination):
Phenol reacts with Br₂ water and decolourizes it whereas alcohols do not react. Phenol reacts with aqueous Br₂ to give electrophilic substitution with immediate formation of a precipitate of 2,4,6-tribromophenol. In this reaction, water acts as a polar solvent.
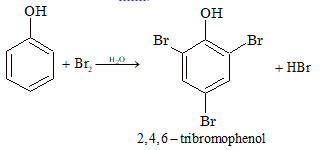
Note: This is the basic reaction for a simple qualitative test for the detection of a phenol.
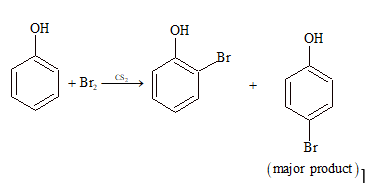
(ii) Sulphonation:
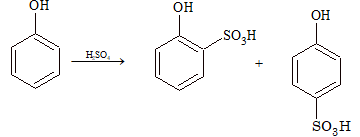
At low temperature (25°C), the ortho-isomer is the major product, whereas at 100°C, it gives mainly the para isomer.
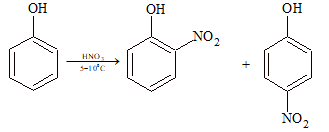
(iii) Nitration:
Phenol reacts with dilute HNO₃ at 5-10°C to form ortho and para nitro phenols, but the yield is poor due to oxidation of phenolic group.
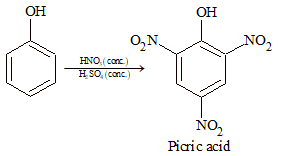
When phenol is treated with concentrated HNO₃ in presence of concentrated H₂SO₄, 2,4,6-trinitrophenol (picric acid) is formed.
(iv) Nitrosation:
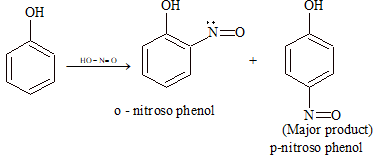
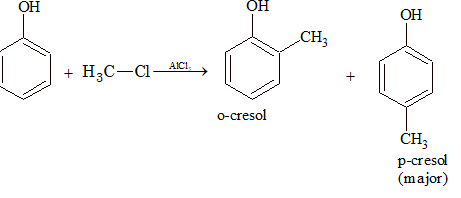
(v) Friedel–Crafts Reaction:
Alkylation:
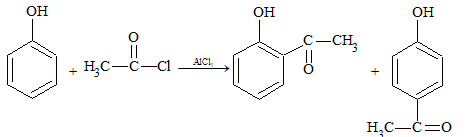
Acylation:
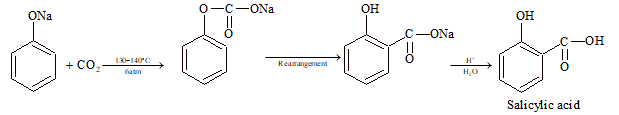
(vi) Kolbe-Schmidt Reaction:
(vii) Reimer-Tiemann Reaction:
Treatment of phenol with chloroform in the presence of aqueous base followed by treatment with aqueous acid gives aldehyde.
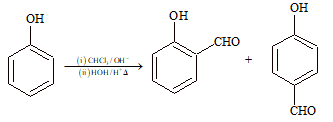
(viii) Reimer-Tiemann Carboxylation:
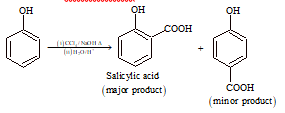
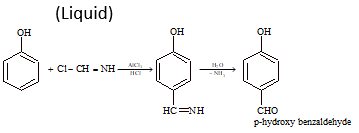
(ix) Gattermann's Reaction:
HCl + H-C≡N (AlCl₃) → Cl-CH=NH (Liquid)
Solved Examples
Problem 1: Acid Dissociation Constants
The correct order of increasing acid dissociation constant of the following compounds is:
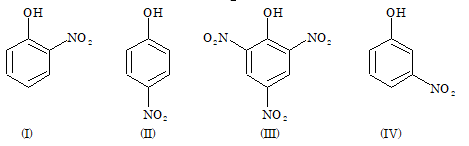
- II < IV < I < III
- IV < III < I < II
- IV < II < I < III
- IV < I < II < III
Solution: (D)
NO₂ group exerts –R effect from o and p position. Therefore, the weakest acid (lowest acid dissociation constant) is compound (IV). More number of nitro group present, more is the acid strength. So, compound (III), due to three nitro group, is the strongest acid with highest acid dissociation constant. Out of I and II, I is weaker than II, due to intramolecular H-bonding. Order: IV < I < II < III
Problem 2: Phthalic Anhydride Reaction
The product in the following reaction:
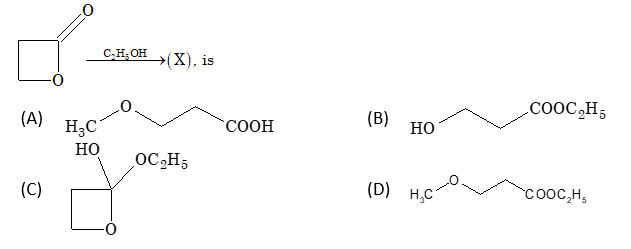
Solution: (B)
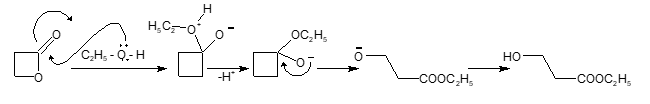
Mechanism: Phthalic anhydride + C₂H₅OH → Monoethyl phthalate One anhydride group opens to form COOC₂H₅ and the other forms COOH
The product is monoethyl phthalate with one ethyl ester group (COOC₂H₅) and one carboxylic acid group (COOH).
Problem 3: Cyclization Reaction
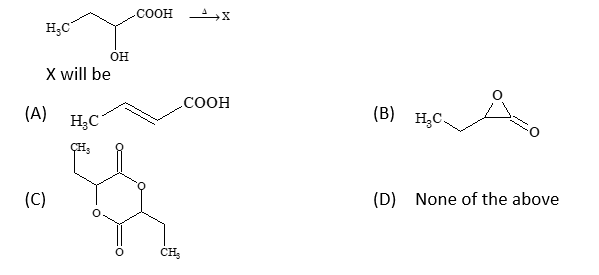
Solution: (C)
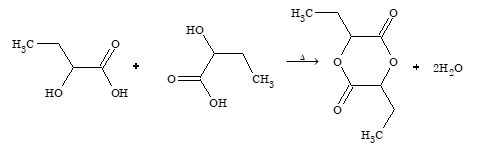
Intramolecular cyclization: CH₃-COOH-OH → Cyclic anhydride + 2H₂O
The compound undergoes intramolecular dehydration to form a cyclic anhydride structure.
Problem 4: Stereochemistry in Nucleophilic Substitution
In which of the following case configuration about chiral carbon is retained?
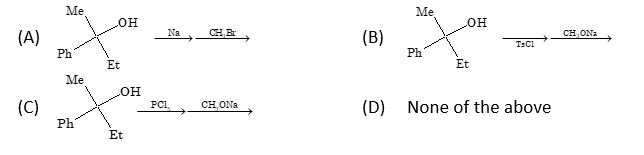
Solution: (A)
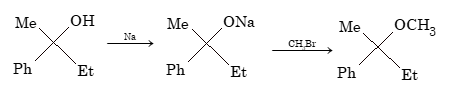
Mechanism for option (A): R-OH + Na → R-ONa + ½H₂ R-ONa + CH₃Br → R-OCH₃ + NaBr (SN2 reaction)
In option (A), the reaction proceeds via alkoxide formation followed by SN2 reaction. The configuration is retained because the C-O bond is not broken during the reaction.
Problem 5: Iodoform Test
An organic compound (A) (molecular formula C₄H₁₀O), upon dehydrogenation gives a compound (B), which forms phenyl hydrazone with phenylhydrazine and both the compounds (A) and (B) respond to iodoform test. Hence, the compound (A) is:
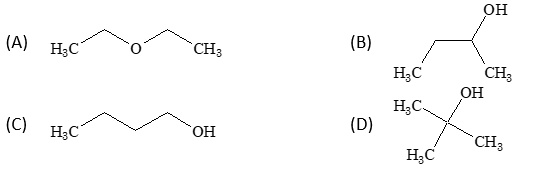
Solution: (B)
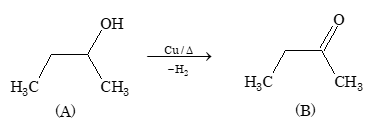
Problem 6: Dehydration and Rearrangement
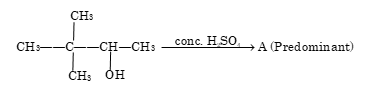
A is:
- (CH₃)₃C-CH=CH₂
- (CH₃)₂C=C(CH₃)₂
- CH₂=C(CH₃)-CH₂-CH₂CH₃
- None of the above
Solution: (B)
Intermediate which is formed during 1,2 methyl shift is 3° carbonium ion. The most stable alkene formed is (CH₃)₂C=C(CH₃)₂ due to maximum substitution (Saytzeff's rule).
Problem 7: Isotope Labeling
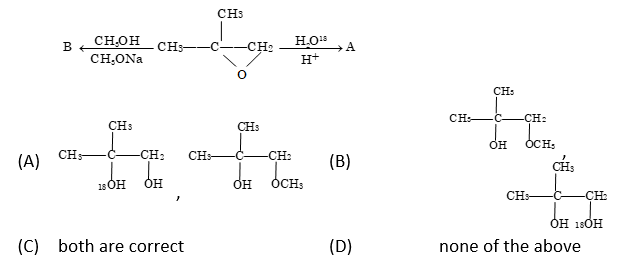
Solution: (A)
As we know stability of carbocation follows following order 3° > 2° > 1°. The ¹⁸O incorporation depends on the reaction pathway and carbocation stability.
Problem 8: Boiling Points of Alcohols
The boiling points of isomeric alcohols follow the order:
- primary > secondary > tertiary
- tertiary > secondary > primary
- secondary > tertiary > primary
- does not follow any order
Solution: (A)
van der Waals forces are responsible for boiling point. Primary alcohols have stronger intermolecular hydrogen bonding due to less steric hindrance, followed by secondary, then tertiary alcohols.
Problem 9: Aromatic Ether Cleavage
The final product obtained in the reaction:
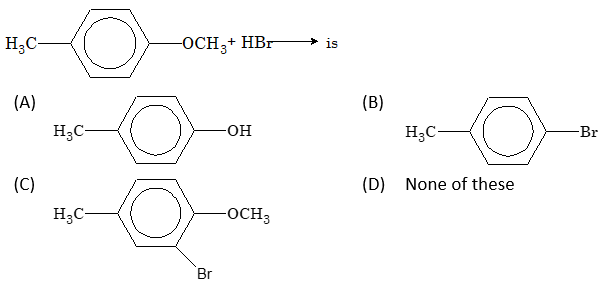
Solution: (A)
The bond between O and sp² hybrid C is strong due to resonance. Therefore, the C-O bond attached to the benzene ring is not easily broken. The product is phenol (C₆H₅-OH).
Problem 10: Oxidation of Secondary Alcohol
The oxidation of 2-hexanol with H₂CrO₄ gives:
- C₂H₅-COOH
- CH₃(CH₂)₂COOH
- CH₃(CH₂)₃-CO-CH₃
- CH₃COOH
Solution: (C)
2° alcohol → Ketone
CH₃(CH₂)₃-CH(OH)-CH₃ → CH₃(CH₂)₃-CO-CH₃
Secondary alcohols on oxidation give ketones, not carboxylic acids.
Problem 11: Phenol Acidity
Which of the following will increase the acidity of phenol?
- NO₂
- CN
- Cl
- All of the above
Solution: (D)
Problem 12: Strongest Acid
The strongest acid among the following aromatic compounds is:
- p-chlorophenol
- p-nitrophenol
- m-nitrophenol
- o-nitrophenol
Solution: (B)
Problem 13: Bromination of Phenol
Phenol reacts with bromide in carbon disulfide solution at low temperature to give as a major product:
- m-bromophenol
- o-and p-bromophenols
- p-bromophenol
- 2, 4, 6 – tribromophenols
Solution: (C)
Problem 14: Phenol vs Carbonic Acid
Phenol does not react with NaHCO₃ because:
- phenol is a weaker acid than carbonic acid
- phenol is a stronger acid than carbonic acid
- phenol is as strong as carbonic acid
- phenol is insoluble in water
Solution: (A)
Problem 15: Fenton's Reagent
Fenton's reagent is:
- H₂O + FeSO₄
- H₂O₂ + FeSO₄
- H₂O₂ + ZnSO₄
- NaOH + FeSO₄
Solution: (B)