Group 13 to 18 elements (except helium), in which the last electron enters the p-orbitals, constitute the p-block. In this chapter will be study systematic group wise details of p-block elements.
Elements: B, Al, Ga, In, Tl.
Trends in Chemical Reactivity and Oxidation States
Most common oxidation states are +3 and +1. Stability of +1 oxidation state increases on going down the group from aluminium to Tl. Thus, in aqueous solution Tl+ is more stable.
Chemical Behaviour
- Metallic character increases down the group due to decreasing I.E. and easy loss of electrons. Boron having small size cannot easily lose its electron and hence is an exception to metallic character.
- Al, Ga, In and Tl having vacant d-orbitals form [M(OH)4]-, [M(OH)4(H2O)2]- and [M(H2O)6]3+ type of complexes with H2O.
- MH3 type of hydrides are formed by group 13 elements. These hydrides act as Lewis acids and form adducts with strong Lewis bases. LiAlH4 is a white crystalline solid obtained as follows:
4LiH + AlCl3Et2O→ LiAlH4 + 3LiCl
- All halides of group 13 elements are known except TlI3. The halides have halogen bridged dimeric structures. Boron trihalides exist as monomers only as boron is too small to co-ordinate with four large halide ions. In case of BF3, the energy required to break p–p bond is not released during bridge formation. The ionic character of trihalides increases on moving down the group due to polarization effects according to Fajan's rule.
- Reaction with alkalies: Boron dissolves to give borates with evolution of H2 gas.
2B + 6NaOH Fuse→ 2Na3BO3 + 3H2Al and Ga with conc. alkalies form metaaluminate and gallate respectively.2Al + 2NaOH + 6H2O → 2Na[Al(OH)4] + 3H2
Sodium metaaluminate
2Ga + 2NaOH + 6H2O → 2Na[Ga(OH)4] + 3H2Sodium gallate
- Oxides: All elements form oxides of formula M2O3, except Tl which forms Tl2O due to inert pair effect.
Boron shows anomalous behaviour due to its small size and high electronegativity. Chemistry of boron is discussed in detail.
BORON
Boron is the only non-metal in its group due to high I.E., it cannot form B3+ instead it forms three covalent bonds.
Properties of Boron
It exists in mainly two allotropic forms, i.e. amorphous dark brown powder and crystalline black hard solid.
Reaction with air:
Boron nitride
Reaction with metals:
Magnesium boride
Boron does not react with H2O.
It reacts with oxidizing agents like HNO3, H2SO4 etc.
Boron reduces CO2 to carbon and SiO2 to Si.
Compounds of Boron
Borax or sodium tetraborate decahydrate (Na2B4O7.10H2O)
Borax naturally occurs as tincal, which contains 50% borax and is obtained from mineral colemanite.
Borax
Its aqueous solution is alkaline due to hydrolysis.
Borax when heated with ethyl alcohol and conc. H2SO4 gives volatile vapours of triethyl borate which burns with green edged flame.
Triethyl borate
On heating, borax loses water of crystallization and swells up to form fluffy mass. On further heating, transparent glassy bead is formed.
Sodium metaborate Boric anhydride
Borax bead
Boric acid or orthoboric acid (H3BO3) or B(OH)3:
It can be obtained from borax or from colemanite as follows,
It behaves as a weak monobasic acid due to the following reaction in water.
With ethyl alcohol and conc. H2SO4, it gives triethyl borate, which burns with a green edged flame.
On heating, boric acid loses water to form B2O3 in three stages.
Illustration 1: Borax structure contains
(A) two BO4 groups and two BO3 groups
(B) four BO4 groups only
(C) four BO3 groups only
(D) three BO4 and one BO3 groups
Solution: (A). Depends on the charge on the anion.
Illustration 2: The order of increasing acidic strength of BF3, BCl3 and BBr3 is
(A) BF3 > BCl3 > BBr3
(B) BF3 < BCl3 < BBr3
(C) BBr3 < BF3 < BCl3
(D) BCl3 > BBr3 > BF3
Solution: (B). Moving down the group, size of halogen increases, hence extent of overlap decreases, so back donation from halogen to boron decreases.
Illustration 3: The bonds present in borazole are
(A) 12σ, 3π
(B) 9σ, 6π
(C) 6σ, 6π
(D) 9σ, 9π
Solution: (A). Borazole is B3N3H6.
Exercise 1
- Inorganic benzene is
(A) BN (B) BF4
(C) B2H6 (D) B3N3H6
- BCl3 does not exist as dimer but BH3 exists as dimer (B2H6) because
(A) chlorine is more electronegative than hydrogen
(B) there is p-p back bonding in BCl3 but BH3 does not contain such multiple bonding
(C) large sized chlorine atoms do not fit in between the smaller boron atoms whereas small sized hydrogen atoms get fitted in between boron atoms
(D) None of the above
COMPOUNDS OF ALUMINIUM
Aluminium chloride (AlCl3):
It can be prepared by passing dry Cl2 or HCl gas over heated Al or by heating a mixture of alumina and carbon in a current of dry chlorine.
It exists as dimer Al2Cl6 in which each Al atom is tetrahedrally surrounded by four Cl atoms.
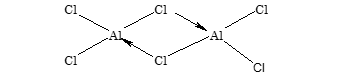
Anhydrous AlCl3 fumes in moist air due to formation of HCl.
It behaves as a Lewis acid and forms adduct with donor molecules. For example,
Alums:
Alums are double salts of formula X2SO4.Y2(SO4)3. 24H2O where X is a monovalent cation and Y is a trivalent cation. For example, K2SO4. Al2(SO4)3. 24H2O is potash alum.
- All alums are Isomorphours.
- All alums form acidic solutions due to formation of H2SO4.
Illustration 4: Al2O3 formation involves large quantity of heat evolution, which makes its use in
(A) deoxidizer
(B) confectionary
(C) indoor photography
(D) thermite welding
Solution: (D).
GROUP 14 ELEMENTS
Elements: C, Si, Ge, Sn, Pb
These elements have valence shell electronic configuration ns2, np2. Common oxidation states observed for group 14 elements are +4 for silicon and +4 and +2 for, Ge, Sn and Pb. Stability of +2 oxidation state increases in the sequence, Ge < Sn < Pb due to inert pair effect.
Trends in Chemical Reactivity
Due to vacant d-orbitals, elements of the group, except carbon, form compounds having co-ordination numbers higher than four like (SiF5)-, (SiF6)2- and (PbCl6)2-.
As we go down the group, the size of atom increases and the interatomic bond strength decreases Thus, the order of catenation decreases as we go down the group.
Tetrahedral, covalent tetrahalides of the type MX4 are formed by Si, Ge, Si and Pb. Ge, Sn and Pb also form dihalides with increasing stability in the sequence, GeX2 < SnX2 < PbX2 due to increasing stability of divalent state going down the group (inert pair).
Oxides of composition MO2 are formed by Si, Ge, Sn and Pb. SiO2 (silica) is a three dimensional network solid of silica and oxygen atoms connected by single covalent bonds with each silicon atom bonded to four oxygen atoms in tetrahedral arrangement. Silica exists both in crystalline and amorphous forms. Monoxides of Sn and Pb are known. Red lead (Pb3O4) is a combination of Pb(II) and Pb(IV) oxides, i.e. 2PbO.PbO2.
Silicates
Silicates are formed by heating metal oxides or metal carbonates with sand, e.g.
Silicates have basic unit of SiO44- where each silicon atom is bonded with four oxide ions tetrahedrally.
Type of silicates:
| Type | Units | Example |
| 1. Orthosilicates | Single SiO44- unit. | ZrSiO4, Mg2SiO4 |
| 2. Pyrosilicates | Two units of SiO44- joined along a corner oxygen. Hence, they are formed of Si2O76- units. | Sc2Si2O7, Zn3(Si2O7)Zn(OH)2.H2O |
| 3. Cyclic silicates | General formula: (SiO32-)n or (SiO3)n2n- | Si3O96- in Ca3Si3O9, Si6O1812-, Be3Al2Si6O18 |
| 4. Chain silicates | (SiO3)n2n- and (Si4O11)n6n- | Li2SiO3, MgSiO3 |
| 5. Two dimensional sheet silicates | (Si2O5)n2n- | Mg(Si2O5)2Mg(OH)2, Al2(OH)4(Si2O5) |
| 6. Three dimensional sheet silicates | All 4 oxygen atoms shared with adjacent SiO44- tetrahedral. | Quartz, zeolites, etc. |
CARBON
It exists in two or more allotropes having different physical properties but identical chemical properties. Amorphous forms are coal, coke and charcoal etc., while crystalline forms are diamond, graphite and fullerenes.
Among group 14 elements, carbon forms pπ – pπ multiple bond easily with itself (in graphite). Hence, carbon shows pronounced ability to form pπ – pπ multiple bonds.
Compounds of Carbon
Oxides:
Carbon forms two stable oxides, i.e. carbon dioxide (CO2) and carbon monoxide (CO). Less stable oxides are C3O2, C5O2 and C12O9 known as suboxides.
Carbon monoxide:
Can be prepared by heating oxalic acid or from formic acid or potassium ferrocyanide as follows:
- H2C2O4H2SO4-H2O→ CO + CO2 + H2O
- HCOOH H2SO4373K→ CO(pure) + H2O
- K4[Fe(CN)6] + 6H2SO4 + 6H2O → 2K2SO4 + FeSO4 + 3(NH4)2SO4 + 6CO
Carbon monoxide reduces metal oxides to metals.
Due to presence of lone pairs of electrons, CO acts as a Lewis base and this is the reason for the formation of metal carbonyls.
Phosgene, a poisonous gas is formed when CO combines with Cl2 in the presence of sunlight
Carbon dioxide:
Carbon dioxide is prepared from calcium carbonate.
Glucose and fructose fermentation also produces CO2
CO2 turns lime water milky due to the formation of insoluble CaCO3 which dissolves by passing excess of CO2 due to soluble Ca(HCO3)2 formation.
In photosynthesis, CO2 is converted into glucose, starch and O2.
Glucose
CO2 is also used in urea formation under a pressure of 220 atm and 453 – 473 K temperature.
Urea
Carbon forms other compounds, namely,
- carbides, e.g. Be2C, Al4C3
- acetylides having (C ≡ C)2- ions, e.g. MgC2, CaC2
- allylide having C34- ions, e.g. Mg2C3
- halides, e,g, CF4, CCl4, CBr4, CFC
- sulphides, e.g. CS, CS2, C3S2
GROUP 15 ELEMENTS
Elements: N, P, As, Sb, Bi.
Nitrogen and phosphorus are non-metals. The metallic character increases down the group due to lower I.E. and larger size. Hence, bismuth shows metallic character. The group state electronic configuration is ns2, np3.
Trends in Chemical Reactivity
Most common oxidation states shown by group 15 elements are – 3, +3 and +5. The stability of highest oxidation state (+5) decreases down the group.
The covalent character goes on decreasing as we move down the group in the sequence, P > As > Sb > Bi. This is due to increasing size of atom which refers to Fajan's rules.
Sb and Bi are the heavier elements of the group and form M3+ cations due to decrease in ionization enthalpy.
In contrast to nitrogen, the size of phosphorus atom forms pπ bonding and forms both cyclic and open chain compounds. Hence, it shows catenation.
NITROGEN
Nitrogen can be obtained from ammonium nitrite, ammonium dichromate, ammonia, urea and barium azide in the laboratory as follows.
Unstable
NH4NO2 → N2 + 2H2O
Barium azide
Nitrogen combines with oxygen under high temperature conditions to form nitric oxide
It combines with H2 in presence of a catalyst at 200 atm and 400 – 500°C temperature to form ammonia.
With metals it forms nitrides.
Some non-metals also combine with nitrogen to form nitrides.
Compounds of Nitrogen
Ammonia:
It is prepared from ammonium salt, magnesium nitride and by Haber's process as follows.
In water it forms a basic solution.
Ammonia reacts with halogens in different stoichiometic ratios.
Explodes in dry state
Ammonia acts as a Lewis base in complex formation.
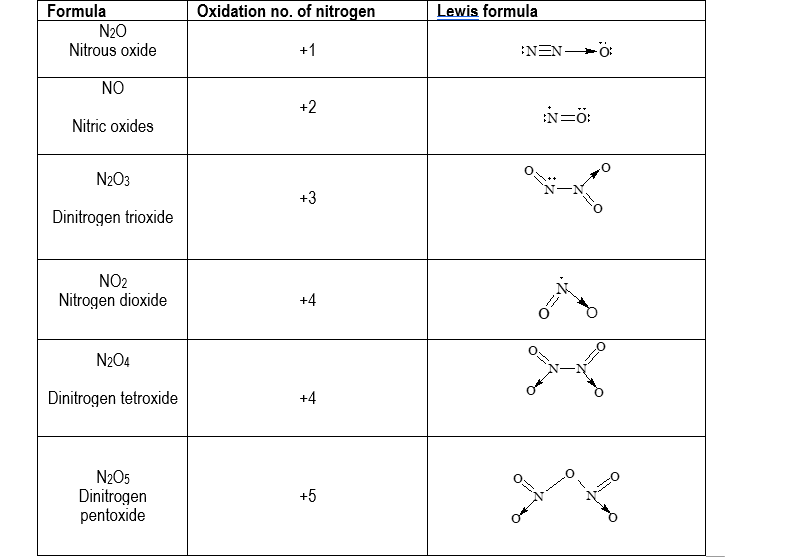
Oxides of nitrogen
Nitrogen forms six different oxides, with oxidation states ranging from +1 to +5. Various oxides with their Lewis formula are listed below:
Preparations of above oxides are as follows:
Chemical reactivity of various oxides can be summarized as follows:
(i) Nitrous oxide is neutral towards litmus and reacts with sulphur, phosphorus, metals and hydrogen as follows:
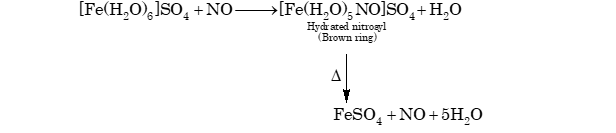
(ii) (a) Nitric oxide is a stable oxide and decomposes only when heated at 800°C.
(b) It dissolves in cold ferrous sulphate solution.
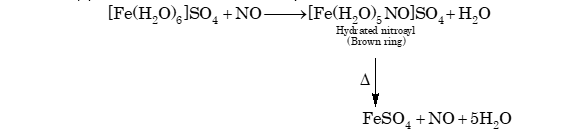
Hydrated nitrosyl
(Brown ring)
(c) It also acts as an oxidizing agent.
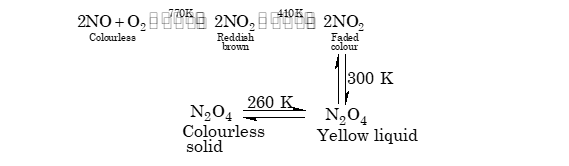
(iii) Nitrogen tetroxide is a reddish brown pungent smelling gas, which associates and dissociates with change in temperature.
NO2 + 2Cu → Cu2O + NO }
(iv) Nitrogen pentoxide (N2O5) is a strong oxidizing agent.
(a) It is decomposed by alkali metals.
(b) With aq. NaCl, the reaction proves that N2O5 exists as ionic nitronium nitrate (NO2+NO3-).
Oxides of nitrogen
Nitrogen forms six different oxides, with oxidation states ranging from +1 to +5. Various oxides with their Lewis formula are listed below:
| Formula | Oxidation no. of nitrogen | Lewis formula |
| N2O Nitrous oxide |
+1 | :N≡N→Ö: |
| NO Nitric oxides |
+2 | :N=Ö: |
| N2O3 Dinitrogen trioxide |
+3 | O \ N—N / O |
| NO2 Nitrogen dioxide |
+4 | N / \ O O |
| N2O4 Dinitrogen tetroxide |
+4 | O O \ / N—N / \ O O |
| N2O5 Dinitrogen pentoxide |
+5 | O ‖ O N O \ / N / \ O O |
Preparations of above oxides are as follows:
Chemical reactivity of various oxides can be summarized as follows:
(i) Nitrous oxide is neutral towards litmus and reacts with sulphur, phosphorus, metals and hydrogen as follows:
(ii) (a) Nitric oxide is a stable oxide and decomposes only when heated at 800°C.
(b) It dissolves in cold ferrous sulphate solution.
Hydrated nitrosyl
(Brown ring)
(c) It also acts as an oxidizing agent.
(iii) Nitrogen tetroxide is a reddish brown pungent smelling gas, which associates and dissociates with change in temperature.
↓
300 K
N2O4
Yellow liquid
↓
260 K N2O4
Colourless
solid
(a) It acts both as oxidizing and reducing agent.
NO2 + 2Cu → Cu2O + NO }
(iv) Nitrogen pentoxide (N2O5) is a strong oxidizing agent.
(a) It is decomposed by alkali metals.
(b) With aq. NaCl, the reaction proves that N2O5 exists as ionic nitronium nitrate (NO2+NO3-).
Oxyacids of nitrogen:
Out of the five oxyacids of nitrogen, nitric acid (HNO3) is most important.
It can be prepared in the lab by heating NaNO3 or KNO3.
It can also be manufactured by Oswald's process.
Reactions of nitric acid are summarized in the following table.
| Concentration | Metal | Main Product |
| Very dilute HNO3 |
Mg, Mn | H2 + M(NO3)x |
| Fe, Zn, Sn | NH4NO3 + M(NO3)x Metal nitrate |
|
| Dilute HNO3 |
Pb, Cu, Ag, Hg | NO + M(NO3)x |
| Fe, Zn | N2O + M(NO3)x | |
| Conc. HNO3 |
Zn, Fe, Pb, Cu, Ag | NO2 + M(NO3)x |
| Sn | NO2 + H2SO4 Metastannic acid |
Oxides of phosphorus:
Phosphorus pentoxide (P4O10) is prepared by burning white phosphorus in excess of air or oxygen.
It acts as a dehydrating agent.
Final product or reaction with water is as follows.
Metaphosphoric Pyrophosphoric Orthophosphoric
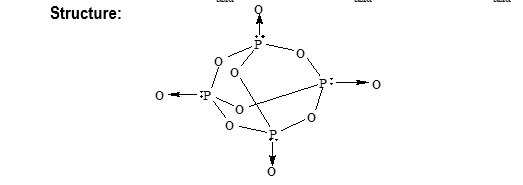
Phosphorous trioxide (P4O6) is prepared by burning white phosphorous in limited supply of air.
It reacts with cold as well as hot water.
It burns in chlorine to form oxy – chlorides.
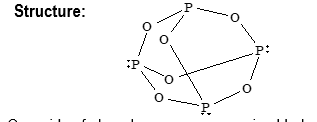
Oxyacids of phosphorus are summarized below:
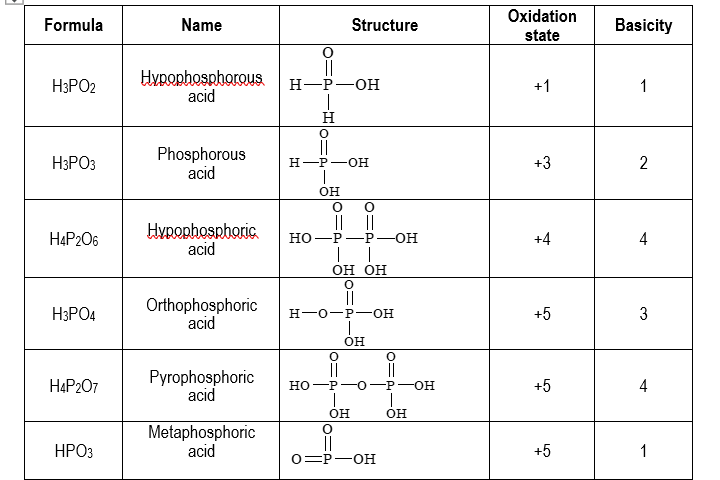
(HPO3)n → It exists in polymeric form.
Exercise 2
- Which is true among the following?
(A) PH3 is stronger base than NH3
(B) Bond angle in PH3 is more than that of NH3
(C) PH3 is stronger reducing agent than NH3
(D) Boiling point of PH3 is more than that of NH3
- Orthophosphoric acid on strong heating forms
(A) P4O10 (B) H4P2O7
(C) (HPO3)x (D) P2O5
GROUP 16 ELEMENTS
Elements: O, S, Se, Te, Po.
First four elements are called chalcogens. The valence shell electronic configuration is ns2, np4, which shows each element has two electrons short of the next noble gas configuration.
Trends in Chemical Reactivity and Oxidation States
- Important oxidation states of S, Se and Te are -2, +2, +4 and +6. Oxidation states +4 and +6 are more pronounced for sulphur and heavier chalcogens, e.g. SF6, SeCl4, Te(OH)6.
- As we go down the group, the electronegativity for S, Se, Te and Po decreases due to increase in size.
- Due to easy loss of electron with increasing size, the metallic character increases on descending down the group. Hence, O, S are non-metals; Se, Te are metalloids whereas Po shows metallic character.
- Tendency for catenation decreases with increasing size as we move down the group.
- Thermal stability of hydrides decreases in the order: H2O > H2S > H2Se > H2Te > H2Po.
- Tendency to form multiple bonds decreases as we go down the group. Thus, S = C = S is quite stable as compared to Se = C = Se, which is unstable and decomposes readily while Te = C = Te is unknown.
OXYGEN
Oxygen can be prepared from various oxides and salts as follows:
Ozone:
In the laboratory ozone can be prepared by passing oxygen through a strong electric field.
Being a metastable allotrope, it always has a tendency to convert back to oxygen.
It acts as a powerful oxidizing agent.
The amount of ozone in a gas mixture can be determined by passing it through KI solution (at constant pH 9.2). The iodine liberated is titrated with sodium thiosulphate (hypo) solution.
O3 layer in the upper atmosphere is destroyed by exhaust gases containing nitrogen oxides. Halogens also damage O3 layer.
Also,
SULPHUR
Sulphur can be extracted from underground deposits by the Frasch process. It can also be prepared from H2S gas as follows:
H2S + 3⁄2O2 → SO2 + H2O
2H2S(g) + SO2(g) — Fe2O3 (catalyst), 673 K —→3⁄8S8(g) + 2H2O(g)
Allotropes of Sulphur
Sulphur exists in three allotropic forms:
- Rhombic sulphur (octahedral sulphur) which exists as S8 molecules. It is a bright yellow solid, soluble in CS2.
- Monoclinic sulphur or β-sulphur is dull yellow and soluble in CS2. Below 369 K it slowly changes to rhombic sulphur. At 369 K (the transition temperature) both forms can coexist.
- Plastic sulphur (amorphous sulphur or γ-sulphur) is a rubber-like, transparent yellow material, insoluble in CS2 and H2O. It is regarded as a super-cooled liquid that exists in long, random, inverted chains of sulphur atoms.
Compounds of Sulphur
Sulphur Dioxide
Among the several oxides of sulphur, SO2 (sulphur dioxide) and SO3 (sulphur trioxide) are the most important.
Preparation
- S8 + 8O2 → 8SO2
- 4FeS2 + 11O2 → 2Fe2O3 + 8SO2
SO2 shows both oxidizing and reducing properties.
Reactions illustrating its dual behaviour
- 2H2S + SO2 → 2H2O + 3S ↓ (oxidizing property of SO2)
- SO2 + 2Mg → 2MgO + S ↓ (oxidizing property of SO2)
- 2KMnO4 + 5SO2 + 2H2O → K2SO4 + 2MnSO4 + 2H2SO4 (SO2 acts as a reducing agent)
- 2FeCl3 + SO2 + 2H2O → H2SO4 + 2FeCl2 + 2HCl (SO2 acts as a reducing agent)
Sulphur Trioxide, Sulphuric Acid & Sodium Thiosulphate
Sulphur trioxide
(SO3) is prepared by catalytic oxidation of sulphur dioxide.
2SO2(g) + O2(g) — V2O5 catalyst, ~723 K —→ 2SO3(g)
It exists in three allotropic forms: α-SO3, β-SO3, γ-SO3.
SO3 is an acidic oxide:
SO3 + H2O → H2SO4 + heat
It forms oleum with concentrated sulphuric acid:
H2SO4 + SO3 → H2S2O7 (oleum)
Sulphuric acid
Sulphur forms many oxyacids; one important acid is sulphuric acid (H2SO4), also called oil of vitriol. It is prepared by the contact process and the lead chamber process.
Contact process (outline)
2SO2 + O2— catalyst —→ 2SO3
H2SO4 + SO3 → H2S2O7 (Oleum)
H2S2O7 + H2O → 2H2SO4
Properties
Sulphuric acid is a low-volatile liquid, a strong acid with a strong affinity for water. It removes water from wet gases that do not react with the acid and dehydrates many organic compounds (e.g., carbohydrates), causing charring.
C12H22O11 + 11 H2SO4 → 12C + 11 H2SO4 + 11 H2O
Both metals and non-metals are oxidized by concentrated sulphuric acid; the acid itself is reduced to SO2.
C + 2H2SO4 → CO2 + 2SO2 + 2H2O
Cu + 2H2SO4 → CuSO4 + SO2 + 2H2O
Sodium thiosulphate
Preparation
Prepared by boiling aqueous solutions of metal sulphites with elemental sulphur.
Na2SO3 + 1⁄8S8— H2O, ~378 K —→ Na2S2O3
It can also be manufactured by Spring’s reaction, treating sodium sulphide and sodium sulphite with iodine:
Na2S + Na2SO3 + I2 → Na2S2O3 + 2NaI
Reaction with acids
Na2S2O3 + 2HCl → 2NaCl + H2O + S ↓ + SO2
Group 17 (Halogens) – Notes & MCQs
Illustrations (MCQs)
(B) it is not a dehydrating agent
(C) it reacts with moisture to give an acid which reacts with NH3 (base)
(D) it is basic while NH3 is acidic
GROUP 17 ELEMENTS (HALOGENS)
Elements: F, Cl, Br, I, At. Astatine is radioactive. The melting and boiling points of halogens increase down the group due to increasing atomic size and hence stronger van der Waals forces. Thus, fluorine and chlorine are pale-yellow and greenish-yellow gases, bromine is a deep reddish-brown liquid, and iodine is a lustrous greyish-black crystalline solid.
Bond energy (X–X) order: F–F < Cl–Cl > Br–Br > I–I. The smaller bond energy of F–F is due to strong lone-pair repulsions in F2. From Cl2 to I2, increasing size reduces effective overlap and the bond strength decreases.
Trends in Oxidation States and Chemical Reactivity
- Fluorine, the most electronegative element, shows oxidation state −1 in all compounds and easily accepts electrons. Halogens act as oxidizing agents and can oxidize halide ions of halogens below them in the group.
Halogens react with metals and non-metals to form halides. Fluorine forms compounds with all elements except He, Ar and Ne.
Halogens form metal halides, M–X, whose ionic character decreases in the order M–F > M–Cl > M–Br > M–I. With increasing halide ion size, polarizability increases, covalent character rises, and ionic character falls. For a metal with multiple oxidation states, the halide of the higher oxidation state is more covalent due to the smaller cation size. Thus, SnCl4, PbCl4 and SbCl5 are more covalent than SnCl2, PbCl2 and SbCl3, respectively.
Hydrogen halides (HF, HCl, HBr, HI) are hydrohalic acids. Only HF is a liquid due to H-bonding. In aqueous solution their acid strength increases in the order HF < HCl < HBr < HI, following the decreasing H–X bond strength.
Other Comparative Properties
- Dipole moment: HI < HBr < HCl < HF.
- Bond length: HF < HCl < HBr < HI.
- Bond strength: HI < HBr < HCl < HF.
- Thermal stability: HI < HBr < HCl < HF.
- Acid strength: HF < HCl < HBr < HI.
- Reducing power: HF < HCl < HBr < HI (HI strongest).
Oxyacids
Halogens form various oxyacids. Fluorine does not form oxyacids. Important oxyacids of chlorine are listed below.
Oxyacids of Chlorine
| Formula | Name | Oxidation no. of Cl |
| HClO | Hypochlorous acid | +1 |
| HClO2 | Chlorous acid | +3 |
| HClO3 | Chloric acid | +5 |
| HClO4 | Perchloric acid | +7 |
Acidic strength: Increases with oxidation number of the halogen: HClO4 > HClO3 > HClO2 > HClO.
Group 17 (Halogens) – Notes & MCQs
Illustrations (MCQs)
(B) it is not a dehydrating agent
(C) it reacts with moisture to give an acid which reacts with NH3 (base)
(D) it is basic while NH3 is acidic
GROUP 17 ELEMENTS (HALOGENS)
Elements: F, Cl, Br, I, At. Astatine is radioactive. The melting and boiling points of halogens increase down the group due to increasing atomic size and hence stronger van der Waals forces. Thus, fluorine and chlorine are pale-yellow and greenish-yellow gases, bromine is a deep reddish-brown liquid, and iodine is a lustrous greyish-black crystalline solid.
Bond energy (X–X) order: F–F < Cl–Cl > Br–Br > I–I. The smaller bond energy of F–F is due to strong lone-pair repulsions in F2. From Cl2 to I2, increasing size reduces effective overlap and the bond strength decreases.
Trends in Oxidation States and Chemical Reactivity
- Fluorine, the most electronegative element, shows oxidation state −1 in all compounds and easily accepts electrons. Halogens act as oxidizing agents and can oxidize halide ions of halogens below them in the group.
Halogens react with metals and non-metals to form halides. Fluorine forms compounds with all elements except He, Ar and Ne.
Halogens form metal halides, M–X, whose ionic character decreases in the order M–F > M–Cl > M–Br > M–I. With increasing halide ion size, polarizability increases, covalent character rises, and ionic character falls. For a metal with multiple oxidation states, the halide of the higher oxidation state is more covalent due to the smaller cation size. Thus, SnCl4, PbCl4 and SbCl5 are more covalent than SnCl2, PbCl2 and SbCl3, respectively.
Hydrogen halides (HF, HCl, HBr, HI) are hydrohalic acids. Only HF is a liquid due to H-bonding. In aqueous solution their acid strength increases in the order HF < HCl < HBr < HI, following the decreasing H–X bond strength.
Other Comparative Properties
- Dipole moment: HI < HBr < HCl < HF.
- Bond length: HF < HCl < HBr < HI.
- Bond strength: HI < HBr < HCl < HF.
- Thermal stability: HI < HBr < HCl < HF.
- Acid strength: HF < HCl < HBr < HI.
- Reducing power: HF < HCl < HBr < HI (HI strongest).
Oxyacids
Halogens form various oxyacids. Fluorine does not form oxyacids. Important oxyacids of chlorine are listed below.
Oxyacids of Chlorine
| Formula | Name | Oxidation no. of Cl |
| HClO | Hypochlorous acid | +1 |
| HClO2 | Chlorous acid | +3 |
| HClO3 | Chloric acid | +5 |
| HClO4 | Perchloric acid | +7 |
Acidic strength: Increases with oxidation number of the halogen: HClO4 > HClO3 > HClO2 > HClO.
Interhalogens & Noble Gases – Notes and MCQs
Reason: This is because the loss of H+ ion from each oxyacid forms the conjugate base as (oxo-anion). The greater the number of oxygen atoms in the ion, the greater is the dispersal of negative charge and hence the higher is the stability of the resulting ion. Hence, the ease of formation of the resulting ions increases accordingly.
Interhalogen Compounds
Halogens can also form interhalogen compounds by reacting with each other. General formula: ABn where n = 1, 3, 5, 7; A = heavier halogen and B = lighter halogen; e.g., ICl3, IF7, etc. All interhalogen compounds are covalent and are generally more reactive than the individual halogens.
The stability of interhalogen compounds increases with the size of the central atom. Shapes of some interhalogen compounds are given below:
| Interhalogen compound | Shape | Hybridization |
| Type AB (n = 1) — BrF, BrCl, IBr, IF | Linear | sp |
| Type AB3 (n = 3) — BrF3, ICl3, IF3 | T-shape | sp3d |
| Type AB5 (n = 5) — BrF5, ICl5, IF5 | Square pyramidal | sp3d2 |
| Type AB7 (n = 7) — IF7 | Pentagonal bipyramidal | sp3d3 |
Pseudo Halide Ions and Pseudo Halogen
Ions consisting of two or more atoms, of which at least one is nitrogen, and having properties similar to halide ions are called pseudohalide ions. Some of these pseudohalide ions can be oxidized to form covalent dimers comparable to halogens. Such dimers are called pseudohalogens; e.g., CN− is a pseudohalide ion and (CN)2 is called cyanogen. SCN− is a pseudohalide ion (thiocyanate) and (SCN)2 is thiocyanogen.
Illustrations (MCQs)
Illustration 8: Which radical can bring about the highest oxidation state of a transition metal?
(A) F− (B) Cl− (C) Br− (D) I−
Solution: (A). F− because it has the smallest size and highest electronegativity.
Illustration 9: Which forms an “acid salt” with base?
(A) HCl (B) HBr (C) HI (D) HF
Sol.: (D). HF exists as H2F2 and forms KHF2 (acid salt) and KF (normal salt).
Illustration 10: Among the fluorides given below which will further react with F2?
(A) NaF (B) CaF2 (C) SF6 (D) IF5
Solution: (D).
Illustration 11: The following acids have been arranged in the order of decreasing acidic strength. Identify the correct order.
(A) ClOH > BrOH > IOH (B) BrOH > ClOH > IOH (C) IOH > BrOH > ClOH (D) ClOH > IOH > BrOH
Solution: (A). Electronegativity of halogen is inversely proportional to basic strength and directly proportional to acidic strength.
Exercise 3
- When Cl2 water is added to an aqueous solution of potassium halide in the presence of CHCl3, a violet colour is obtained. On adding more Cl2 water, the violet colour disappears and a colourless solution is obtained. This test confirms the presence of which in the aqueous solution?
(A) Iodide (B) Bromide (C) Chloride (D) Iodide and Bromide - Which of the following species is linear?
(A) I3− (B) XeF2 (C) ICl2− (D) All of the above
GROUP 18 ELEMENTS
Elements: He, Ne, Ar, Kr, Xe, Rn. These are noble gases. Their valence shells are fully occupied (ns2 np6), so gain or loss of electrons is difficult and they are chemically least reactive. All noble gases are monoatomic.
The natural abundance of noble gases in dry air is around 1%, of which argon is the major component. The main commercial source of He is natural gas. Radon is obtained as the decay product of the Radium-226 isotope.
Isolations: Rare gases are isolated from air by Ramsay and Rayleigh’s method. Several xenon compounds with very electronegative elements like fluorine and oxygen have been synthesized, while no true compounds of He, Ne and Ar are known. Krypton forms compounds rarely (notably KrF2). This trend is explained by increasing atomic size down the group, decreasing ionization energy, and the availability of vacant d-orbitals.
Xenon Fluoride and Xenon Oxygen Compounds
XeF2, XeF4 and XeF6 are the main fluorides of xenon.
XeFx are stable only in Ni containers. They are strong fluorinating agents and are hydrolysed even by traces of water. Xenon fluorides react with fluoride-ion acceptors to form cationic species and with fluoride-ion donors to form fluoroanions.
Hydrolysis of xenon fluorides produces xenon oxygen compounds. XeO3 with aqueous alkali forms hydrogen xenate ion.
Structure of xenon compounds
| Compound | Structure | Hybridization |
| XeF2 | Linear | sp3d |
| XeF4 | Square planar | sp3d2 |
| XeF6 | Distorted octahedron | sp3d3 |
| XeO3 | Pyramidal | sp3 |
| XeOF4 | Square pyramidal | sp3d2 |
| XeO4 | Tetrahedral | sp3 |
| XeO2F2 | Distorted octahedron | sp3d |
Separation of Rare Gases
Rare gases are separated individually from their mixture by Dewar’s charcoal adsorption method. After removing O2, CO2 and N2 from dry air, the remaining mixture of rare gases is adsorbed by coconut-charcoal at about −100 °C in a Dewar flask.
Illustration 12 :
Geometry of XeOF4 molecule is
(A) square planar (B) square pyramidal
(C) triangular bipyramidal (D) distorted octahedron
Solution. (B). It involves sp3d2 hybridization with one lone pair.
Exercise 4
i) The case of liquefaction of noble gases decreases in the order
(A) He > Ne > Ar > Kr > Xe (B) Xe > Kr > Ar > Ne > He
(C) Kr > Xe > He > Ar > Me (D) Ar > Kr > Xe > He > Me
ii) The gaseous mixture used by divers for respiration is
(A) N2 + O2 mixture (B) He + O2 mixture
(C) Ar + O2 mixture (D) Ne + O2 mixture
ANSWER TO EXERCISE
Exercise 1. 1) D 2) C
Exercise 2. 1) C 2) D
Exercise 3. 1) A 2) D
Exercise 4. 1) B 2) B
SOLVED EXAMPLES
1. Alumina on heating with carbon in nitrogen atmosphere gives
(A) Al + CO (B) Al + CO2
(C) AlN + CO (D) Al + CO + N2
Sol. (C).
2. Thallium shows different oxidation states
(A) as it is a transition metal (B) due to inert pair effect
(C) because of its amphoteric character (D) due to its high reactivity
Sol. (B).
3. Aluminum vessels should not be washed with materials containing washing soda because
(A) washing soda is expensive
(B) washing soda is easily decomposed
(C) washing soda reacts with aluminum to form soluble aluminates
(D) washing soda reacts with aluminum to form insoluble aluminum oxide
Sol. (C).
4. Which of the following is pseudoalum?
(A) (NH4)2SO4·Fe2(SO4)3·24H2O (B) K2SO4·Al2(SO4)3·24H2O
(C) MnSO4·Al2(SO4)3·24H2O (D) None of the above
Sol. (C).
5. Which is used in high temperature thermometry?
(A) Na (B) Tl (C) Ga (D) Hg
Sol. (C).
6. The main factor responsible for weak acidic nature of B–F bonds in BF3 is
(A) large electronegativity of fluorine
(B) three centred two electron bonds in BF3
(C) pπ–dπ back bonding
(D) pπ–pπ back bonding
Sol. (D).
7. Which of the following is methanide?
(A) Be2C (B) Al4C3 (C) Mg2C3 (D) Both (A) and (C)
Sol. (D). Both Be2C and Al4C3 are called methanides because they react with water to give methane.
8. Which of the following nitrate will produce laughing gas on heating?
(A) NaNO3 (B) Ca(NO3)2 (C) Hg(NO3)2 (D) NH4NO3
Sol. (D).
9. Among the following oxides, least acidic is
(A) P4O6 (B) P4O10 (C) As4O6 (D) As4O10
Sol. (C). Trioxides are less acidic than pentoxides. Acidic character decreases down the group.
10. The equivalent weight of phosphoric acid (H3PO4) in the reaction — is
(A) 25 (B) 49 (C) 59 (D) 98
Sol. (D).
ASSIGNMENT PROBLEMS
1. Which of the following has the minimum heat of dissociation?
(A) (B) (C) (D)
2.The role of fluorspar (CaF2) which is added in small quantities in the electrolytic reduction of alumina dissolved in fused cryolite (Na3AlF6) is
(A) as a catalyst
(B) to make the fused mixture very conducting
(C) to increase the temperature of the melt
(D) to decrease the rate of oxidation of carbon at the anode
3. The dissociation of Al(OH)3 by a solution of NaOH results in the formation of
(A) (B) (C) (D)
4. B–H–B bridge in B2H6 is formed by the sharing of
(A) 2 electrons (B) 4 electrons (C) 1 electron (D) 3 electrons
5. Fluorine is more electronegative than both boron and phosphorus. What conclusion can be drawn from the fact that BF3 has no dipole moment but PF3 does?
(A) BF3 is not spherically symmetrical
(B) BF3 must be triangular planar
(C) BF3 must be linear
(D) Atomic radius of P is larger than atomic radius of B
6. Identify the reagent I and II respectively in the following sequence:
[sequence]
(A) Acid, Al (B) Acid, C (C) Acid, Fe (D) Acid, Mg
7. The structure of quartz, mica and asbestos have same common units of
(A) (SiO4)4− (B) (SiO3)2− (C) (SiO4)2− (D) SiO2
8. Antidote for CO poisoning is
(A) Carborundum (B) Pure CO2 (C) Carbogen (D) Carbonyl chloride
9. R3SiCl on hydrolysis forms
(A) R3SiOH (B) R3Si–O–SiR3 (C) R3Si=O (D) None of the above
10. The thermal stability order for group 14 halides is
(A) CX4 > SiX4 > GeX4 > SnX4
(B) SnX4 > GeX4 > SiX4 > CX4
(C) SiX4 > CX4 > GeX4 > SnX4
(D) None of the above
11. Molecular shapes of SF4, CF4 and XeF4 are
(A) same with 2, 0 and 1 lone pair of electrons respectively
(B) same with 1, 1 and 1 lone pair of electrons respectively
(C) different with 0, 1 and 2 lone pair of electrons respectively
(D) different with 1, 0 and 2 lone pair of electrons respectively
12. The number of moles of water required to completely hydrolyze one mole of P4O10 is
(A) two (B) three (C) four (D) six
13. Ammonium dichromate is used in some fireworks. The green coloured powder blown during explosion is
(A) CrO3 (B) Cr2O3 (C) Cr (D) CrO(O2)
14. Antichlor is a compound
(A) which absorbs chlorine
(B) which removes chlorine from a material
(C) which liberates Cl2 from bleaching powder
(D) which acts as a catalyst in the manufacture of chlorine
15. Which of the following is not a fertilizer?
(A) 4Ca(H2PO4)2
(B) 3Ca(H2PO4)2 + 7CaSO4 + 2HF
(C) Ca(NO3)2 + NH4NO3
(D) CaF2 + (NH4)3PO4
ANSWERS TO ASSIGNMENT PROBLEMS
1. D 2. B 3. B 4. A 5. B 6. D
7. A 8. C 9. B 10. A 11. D 12. D
13. B 14. B 15. B