Transition Elements & Coordination Compounds – Study Notes, Properties & Examples
Master the concepts of Transition Elements and Coordination Compounds with our comprehensive guide designed for students preparing for IIT-JEE, NEET, and competitive exams. From the general properties of d-block elements to the advanced theories of bonding, this page covers everything you need in one place.
What are Transition Elements?
Transition elements are d-block elements with a partially filled d-subshell in their atoms or common oxidation states. They are located between the s-block and p-block in the periodic table and exhibit properties intermediate between the two.
Examples: Iron (Fe), Copper (Cu), Nickel (Ni).
Note: Elements like Zn, Cd, and Hg are in the d-block but are not true transition elements due to fully filled d-orbitals (d10 configuration).
General Properties of Transition Metals
Transition metals are known for:
- High melting and boiling points due to strong metallic bonding.
- Variable oxidation states (e.g., Fe2+ and Fe3+).
- Formation of coloured compounds because of d–d electronic transitions.
- Magnetic properties like paramagnetism and ferromagnetism.
- Ability to form complex ions with ligands via coordinate bonds.
- Catalytic activity in industrial reactions (e.g., V2O5 in Contact Process).
Compounds of Transition Metals
Some important examples include:
- Potassium Permanganate (KMnO4): Strong oxidizing agent in acidic, neutral, and alkaline mediums.
- Potassium Dichromate (K2Cr2O7): Used in volumetric analysis and oxidation reactions.
- Copper Sulphate (CuSO4·5H2O): Blue crystalline solid, used in agriculture and electroplating.
- Silver Nitrate (AgNO3): Known as lunar caustic, used in photography and analytical chemistry.
Coordination Compounds
Definition: Complexes in which a central metal ion is bonded to molecules/ions (ligands) via coordinate bonds.
Key Terms:
- Central Metal Ion – metal atom accepting electron pairs.
- Ligands – neutral or anionic species donating lone pairs.
- Coordination Number (C.N.) – number of donor atoms directly bonded to the central metal.
- Oxidation Number – hypothetical charge on the metal if ligands are removed.
Examples: [Co(NH3)6]3+, [Fe(CN)6]4-, [Cu(NH3)4]2+.
IUPAC Nomenclature of Coordination Compounds
- Cations before anions in naming.
- Ligands are named alphabetically, with prefixes (di, tri, tetra).
- Anionic complexes end in -ate (e.g., ferrate, chromate).
- Oxidation state is given in Roman numerals.
Example:
[Cr(H2O)4Cl2]Cl → tetraaquadichlorochromium(III) chloride
Isomerism in Coordination Compounds
Classes include:
- Structural Isomerism: Ionization, hydrate, linkage, and coordination isomerism.
- Stereoisomerism: Geometrical (cis–trans) and optical isomerism.
Crystal Field Theory (CFT)
Explains splitting of d-orbitals in different ligand geometries:
- Octahedral complexes – splitting into t2g (low energy) and eg (high energy) orbitals.
- Tetrahedral complexes – reverse splitting order.
- Determines high-spin vs. low-spin configurations depending on ligand strength (Spectrochemical Series).
Why Study with Us?
- Exam-focused explanations based on FIITJEE and NCERT patterns.
- Comprehensive notes for transition elements and coordination compounds.
- Detailed solved examples and exercises for practice.
- Prepared for IIT-JEE, NEET, and other competitive exams.
INTRODUCTION
1. The elements in which the differentiating electron enters into the penultimate d-orbitals are called d-block elements.
2. The elements with partly filled d-subshell in their atoms or in common oxidation states are known as transition elements.
3. The d-block elements are present in between those of s-block and p-block elements. Because these elements show properties intermediate between the properties of s and p-block elements.
4. The elements of group IB, IIB, IIIB, IVB, VB, VIB, VIIB and VIIIB of periodic table are called transition elements.
5. The general electronic configuration of transition elements are (n-1)d1-10ns0-2.
6. The elements with ns2(n-1)d10 configuration are not transition elements although they belong to d-block, e.g. Zn(3d104s2), Cd(4d105s2), Hg(5d106s2).
7. Cu, Ag and Au are called coinage metals.
8. Zn, Cd and Hg are the last members of each d-block series.
9. First transition series starts from Sc (Z = 21) to Zn (Z = 30) are called 3d-series.
10. Second transition series starts from Y (Z = 39) to Cd (Z = 48) are called
4d-series.
11. Third transition series starts from La (Z=57) and followed by Hf (Z=72) to Hg (Z=80) are called 5d-series.
12. Elements of VIIIB group (Fe, Co, Ni, Ru, Rh, Pd, Os, Ir, Pt) are called typical transition elements.
GENERAL PROPERTIES OF TRANSITION ELEMENTS
Metallic Character
All the transition elements are metals. They are less metallic than alkali and alkaline earth metals. In a transition series on moving from left to right, the strength of metallic bond first increases and then decreases.
Conductivity
All the transition metals are good conductors of heat and electricity. Silver is the best conductor of electricity.
Density
Because of small size of their atoms and strong metallic bonding the density and hardness of transition elements are high.
Melting and Boling Points
These elements have high melting and boiling points due to strong interatomic attraction and strong metallic bonding. In a transition series the melting point first increases and then decreases. Melting point of Zn, Cd and Hg are low because of weak metallic bonding in them.
Atomic and Ionic Radii
The atomic and ionic radii of transition elements are smaller than those of s-block elements and larger than those of p-block elements.
Atomic and ionic radii ∝ 1effective nuclear charge
∝ Screening effect
∝ Inter-electronic repulsion
Ionization Energy
The ionization energy (IE) of transition elements are higher than those of s-block elements but lower than p-block elements. In a particular transition series, ionization energy although increases gradually as we move from left to right but this increase is not appreciable.
The increase in ionization energy is due to increase in nuclear charge, the effect of increase in nuclear charge is partly balanced by the increase in screening effect. Consequently, the increase in ionization energy along the period of d-block elements is very small.
Coloured Complexes
The d-orbitals in the transition elements do not have same energy in their complexes.
(i) Under the influence of the ligands attached, the d-orbitals split into two sets of orbitals having slightly different energies.
(ii) In the transition elements which have partially filled d-orbitals, the transition of electrons can take place from one of the lower energy d-orbitals to some higher energy d-orbitals within the same subshell. The energy required for this transition falls in the visible region. So, when white light falls on these complexes they absorb a particular colour from the radiation for the promotion of electron and the complementary colours are emitted. The colour of the complex is due to this emitted radiation.
Magnetic Properties
(i) Diamagnetic substances are feebly repelled by an applied magnetic field. Such substances have no unpaired electrons.
(ii) Paramagnetic substances are attracted by an applied magnetic field. Such substances have at least one unpaired electron.
(iii) Ferromagnetism is a special type of paramagnetism in which permanent magnetic moment is acquired by the substance due to the presence of unpaired electrons.
(iv) The magnetic moment arises from the spin and orbital motions in ions or molecules. It is given as:
u = root n (n + 2) Bohr magneton Where n is number of unpaired electrons.
Formation of Alloy
Since d-block elements have almost similar atomic size, they can mutually substitute one another in the crystal lattice to form alloys, e.g. german silver (Cu, Ni, Zn), nickel steel (2.5 – 5.0% Ni), tungsten steel (6-8% W).
Complex Ion Formation
All transition elements form co-ordination compounds readily. The tendency of the transition metals to form complexes is due to
(i) small size of the metal ions.
(ii) large ionic or nuclear charge.
(iii) available vacant d-orbitals.
(iv) low basicity of the metal ions.
Interstitial Compounds
Transition metals can trap some small atoms like hydrogen, boron, carbon, nitrogen etc. in vacant spaces in their crystal lattice forming interstitial compounds. Carbon and nitrogen always occupy octahedral holes. Hydrogen is smaller and always occupies tetrahedral holes. The composition of these compounds is generally non-stoichiometric, e.g. TiH1.73, PdH0.56, VH0.56 but may approach regular stoichiometry and a regular structure, e.g. TiC and VN. The later transition elements of the first series form non-stoichiometric carbides with irregular structures. Such as Cr7C3, which are more reactive than the interstitial carbides of the early transition elements.
COMPOUNDS OF TRANSITION METALS
Potassium Permanganate (KMnO4)
Preparation
It is prepared by fusing pyrolusite ore either with KOH or K2CO3 in presence of atmospheric oxygen.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
2MnO2 + 2K2CO3 + O2 → 2K2MnO4 + 2CO2
The fused mass is extracted with water and the solution is treated with a current of Cl2 or O3 or CO2.
2K2MnO4 + Cl2 → 2KMnO4 + 2KCl
2K2MnO4 + H2O + O3 → 2KMnO4 + 2KOH + O2
2K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3
Properties
It is a purple coloured crystalline compound, fairly soluble in water.
Effect of heat
2KMnO4 —Δ→ K2MnO4 + MnO2 + O2
4KMnO4 + 4KOH —Δ→ 4K2MnO4 + 2H2O + O2
Reaction with conc. H2SO4
On treatment with conc. H2SO4 it forms manganese heptoxide via permanganyl sulphate which decomposes explosively on heating.
2KMnO4 + 3H2SO4 → 2KHSO4 + (MnO3)2SO4 + 2H2O
(MnO3)2SO4 + H2O → Mn2O7 + H2SO4
Mn2O7 → 2MnO2 + 3⁄2 O2
Oxidizing behaviour
Potassium permanganate acts as an oxidizing agent in alkaline, neutral or acidic solution.
- In alkaline solution: 2KMnO4 + 2KOH → 2K2MnO4 + H2O + [O]
- In neutral solution: 2KMnO4 + H2O → 2MnO2 + 2KOH + 3[O]
- In acidic solution: 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5[O]
The important oxidation reactions are
(a) In acidic medium:
-
2MnO4− + 10Fe2+ + 16H+ → 10Fe3+ + 2Mn2+ + 8H2O
- 2MnO4− + 10I− + 16H+ → 2Mn2+ + 5I2 + 8H2O
- 5H2C2O4 + 2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 10CO2 + 8H2O
- 2KMnO4 + 5SO2 + 2H2O → K2SO4 + 2MnSO4 + 2H2SO4
- 2KMnO4 + 3H2SO4 + 5H2S → K2SO4 + 2MnSO4 + 5S + 8H2O
- 2KMnO4 + 3H2SO4 + 10HX → K2SO4 + 2MnSO4 + 8H2O + 5X2
(b) In neutral medium:
- 2KMnO4 + 3H2S → 2KOH + 2MnO2 + 2H2O + 3S
- 2KMnO4 + 3Na2S2O3 + H2O → 2KOH + 2MnO2 + 3Na2SO4 + 3S
(c) In alkaline medium:
-
2KMnO4 + KI + H2O → 2KOH + 2MnO2 + KIO3
- Alkene + H2O + [O] (alk. KMnO4) → vicinal diol (–CH2OH–CH2OH)
Potassium Dichromate (K₂Cr₂O₇)
(i) It is an orange-red coloured crystalline compound.
(ii) It is moderately soluble in cold water but freely soluble in hot water. It melts at 398°C.
Effect of heat
(i)
2K₂Cr₂O₇ —Δ→ 2K₂CrO₄ + Cr₂O₃ + 3/2 O₂ (orange) (yellow)
(ii)
K₂Cr₂O₇ + 2KOH —Δ→ 2K₂CrO₄ + H₂O (orange) (yellow)
On acidifying yellow colour again changes to orange.
2CrO₄²⁻ + 2H⁺ → 2Cr₂O₇²⁻ + H₂O (yellow) (orange)
Chromyl chloride test (test of chloride ion)
When a mixture of a metal chloride and potassium dichromate is heated with conc. H₂SO₄, orange-red vapours of chromyl chloride are evolved.
K₂Cr₂O₇ + 6H₂SO₄ + 4NaCl → 2KHSO₄ + 4NaHSO₄ + 2CrO₂Cl₂ + 3H₂O (chromyl chloride)
When chromyl chloride vapours are passed through NaOH solution, yellow coloured solution is obtained.
CrO₂Cl₂ + 4NaOH → Na₂CrO₄ + 2NaCl + 2H₂O (yellow solution)
When lead acetate and acetic acid are added into this yellow solution, a yellow precipitate is obtained.
Na₂CrO₄ + (CH₃COO)₂Pb —CH₃COOH→ PbCrO₄ + 2CH₃COONa (yellow ppt.)
Ferrous Sulphate (FeSO4·7H2O) or Green Vitriol
It is also known as harakasis.
Preparation
Commercially, it is prepared by the slow oxidation of iron pyrites in the presence of air and moisture.
2FeS2 + 2H2O + 7O2 → 2FeSO4 + 2H2SO4
On crystallization, green crystals are formed.
Properties
Due to atmospheric oxidation the crystals acquire brownish yellow colour due to formation of basic ferric sulphate.
4FeSO4 + 2H2O + O2 → 4Fe(OH)·SO4
basic ferric sulphate
(i) Action of heat
FeSO4·7H2O — 300°C, −7H2O → 2FeSO4 → high temperature → Fe2O3 + SO2 + SO3
(green) → (white)
(ii) It reduces gold chloride to gold
AuCl3 + 3FeSO4 ⟶ Au + Fe2(SO4)3 + FeCl3
(iii) It combines with KCN (excess) forming potassium ferrocyanide.
FeSO4 + 2KCN ⟶ Fe(CN)2 + K2SO4
Fe(CN)2 + 4KCN ⟶ K4Fe(CN)6
FeSO4 + 6KCN ⟶ K4[Fe(CN)6] + K2SO4
(iv) A cold solution of FeSO4 absorbs nitric oxide forming dark brown addition compound.
FeSO4 + NO ⟶ FeSO4.NO
nitroso ferrous sulphate (brown)
Silver Nitrate (AgNO3) or Lunar Caustic
Preparation
3Ag + 4HNO3 (dil.) —Δ—> 3AgNO3 + NO + 2H2O
Properties
It is a colourless crystalline compound soluble in water and alcohol.
On heating above its melting point, it decomposes.
2AgNO3 ⟶ 2AgNO2 + O2
When heated to red heat, it further decomposes to metallic silver.
2AgNO3 ⟶ 2Ag + 2NO2 + O2
| Reaction | Equation |
|---|---|
| Halides | AgNO3 + NaX ⟶ AgX↓ + NaNO3 |
| Phosphate | 3AgNO3 + Na3PO4 ⟶ Ag3PO4↓ + 3NaNO3 |
| Sulphide | 2AgNO3 + Na2S ⟶ Ag2S↓ + 2NaNO3 (black ppt.) |
| Chromates | 2AgNO3 + K2CrO4 ⟶ Ag2CrO4↓ + 2KNO3 (brick red ppt.) |
| Thiocyanates | AgNO3 + NaCNS ⟶ AgCNS↓ + NaNO3 |
| Thiosulphate | 2AgNO3 + Na2S2O3 ⟶ Ag2S2O3↓ + 2NaNO3 (white ppt.) Ag2S2O3 + H2O ⟶ Ag2S↓ + H2SO4 (black) |
| Tollen's reagent | 2AgNO3 + 2NH4OH ⟶ Ag2O↓ + 2NH4NO3 + H2O Ag2O + 2NH4NO3 + 2NH4OH ⟶ 2[Ag(NH3)2]NO3 + 3H2O |
Reactions Involving Silver Compounds (Photographic Film)
Reactions Involving Silver Compounds (Photographic Film)
It converts glucose to gluconic acid.
It oxidizes HCHO to HCOOH.
Ag₂O + HCHO → 2Ag + HCOOH
The reaction used in photographic film
The treatment of the exposed photographic film with a reducing agent is called developing of the film, and the chemicals used for developing as reducing agents are called developers (e.g., potassium ferrous oxalate, or an alkaline solution of pyrogallol or an alkaline solution of quinol).
Quinol + 2AgBr → Quinone + 2Ag + 2HBr
In order to make the image permanent, it is necessary to remove the unreduced AgBr from the surface of the developed film, known as fixing of the image. This is done by dipping the developed film in hypo solution (sodium thiosulphate).
AgBr + 2Na2S2O3 → Na3[Ag(S2O3)2] + NaBr
(sodium argento thiosulphate)
Cuprous Chloride (Cu₂Cl₂)
Preparation
-
By passing SO₂ through the solution containing copper sulphate and sodium chloride.
2CuSO₄ + 2NaCl + 2H₂O + SO₂ → Cu₂Cl₂ + Na₂SO₄ + 2H₂SO₄
- By boiling copper sulphate solution with excess of copper turnings in the presence of HCl.
CuSO₄ + 2HCl → CuCl₂ + H₂SO₄
CuCl₂ + Cu → Cu₂Cl₂
Properties
It is a white solid, insoluble in water.
Cu₂Cl₂ + 4HCl → 2H₂CuCl₃ Cu₂Cl₂ + 6HCl → 2H₃CuCl₄ Cu₂Cl₂ + 2H₂O + O₂ → 2[CuCl₂·Cu(OH)₂] (basic cupric chloride) Cu₂Cl₂ + 2NaOH → Cu₂O (yellow, changing to red) + 2NaCl + 2H₂O Cu₂Cl₂ + H₂S → Cu₂S + 2HCl
Copper Sulphate (CuSO₄·5H₂O) or Blue VitriolPreparation
- CuO + H₂SO₄ → CuSO₄ + H₂O
- Cu(OH)₂ + H₂SO₄ → CuSO₄ + 2H₂O
- Cu(OH)₂, CuCO₃ + 2H₂SO₄ → 2CuSO₄ + 3H₂O + CO₂
Properties
It is a blue crystalline compound and is partially soluble in water.
- Effect of heating
- CuSO₄·5H₂O (blue) → (100°C) CuSO₄·3H₂O (pale blue)
- CuSO₄·3H₂O → (200°C) CuSO₄·H₂O (bluish white)
- CuSO₄·H₂O → (220°C) CuSO₄ (white)
- CuSO₄ —(720°C)—> CuO + SO₃
- SO₃ → SO₂ + ½ O₂
- Action of NH₄OH
- CuSO₄ + 2NH₄OH → Cu(OH)₂↓ + (NH₄)₂SO₄
- Cu(OH)₂ + 2NH₄OH + (NH₄)₂SO₄ → [Cu(NH₃)₄]SO₄ + 4H₂O
- The complex formed is known as Schweizer’s reagent. It is used in the cuprammonium process for the manufacturing of rayon.
- Action of potassium ferrocyanide
- Reddish brown precipitate of cupric ferrocyanide is formed (test of Cu²⁺ ion).
- 2CuSO₄ + K₄[Fe(CN)₆] → Cu₂[Fe(CN)₆] + 2K₂SO₄
- Action of potassium thiocyanate
- CuSO₄ + 2KCNS → Cu(CNS)₂ + K₂SO₄
- 2CuSO₄ + 2KCNS + SO₂ + 2H₂O → Cu₂(CNS)₂ + K₂SO₄ + H₂SO₄
- (cupric thiocyanate)
- Action of potassium iodide
- [CuSO₄ + 2KI → CuI₂ + K₂SO₄] × 2
- 2CuI₂ → Cu₂I₂ + I₂
- 2CuSO₄ + 4KI → Cu₂I₂ + 2K₂SO₄ + I₂
Zinc Sulphate (ZnSO4.7H2O) or White Vitriol Preparation
Zn + H2SO4 → ZnSO4 + H2
ZnO + H2SO4 → ZnSO4 + H2O
ZnCO3 + H2SO4 → ZnSO4 + H2O + CO2
The solution on concentration and crystallization below 39°C gives colourless crystals of zinc sulphate (ZnSO4.7H2O)
Properties
It is a colourless, crystalline solid. It is an efflorescent substance and easily soluble in water.
- Action of Heat:
ZnSO₄·7H₂O →39-70°C ZnSO₄·6H₂O →70°C ZnSO₄·H₂O →280°C ZnSO₄ (anhydrous) →800°C ZnO + SO₃ + O₂ - ZnSO₄ + 2NaOH → Zn(OH)₂↓ + Na₂SO₄
White precipitate of Zn(OH)₂ dissolves in excess of NaOH.
Zn(OH)₂ + 2NaOH → Na₂ZnO₂ + 2H₂O (sodium zincate) - 4ZnSO₄ + 4Na₂CO₃ + 3H₂O → ZnCO₃·3Zn(OH)₂ (basic zinc carbonate) + 4Na₂SO₄ + 3CO₂
- ZnSO₄ + 2NaHCO₃ → ZnCO₃ + Na₂SO₄ + H₂O + CO₂
- With alkali metal sulphates and (NH₄)₂SO₄, it forms double sulphates such as K₂SO₄·ZnSO₄·6H₂O
- It is used for the preparation of lithopone (ZnS + BaSO₄), which is also known as Charlton white and used as a pigment for white paints.
Mercurous chloride (Hg₂Cl₂) or Calomel
Preparation
- Hg₂(NO₃)₂ + 2HCl → Hg₂Cl₂ + 2HNO₃
- Hg₂(NO₃)₂ + 2NaCl → Hg₂Cl₂ + 2NaNO₃
- Hg + HgCl₂ → Hg₂Cl₂
- 2HgCl₂ + 2H₂O + SO₂ → Hg₂Cl₂ + H₂SO₄ + 2HCl
Properties
It is an amorphous, odourless white powder. It is sparingly soluble in water. It sublimes at 373°C.
- It dissolves in chlorine water, aqua regia or a mixture of KClO₃ and concentrated HCl.
Hg₂Cl₂ + 2Cl₂ → 2HgCl₂ - Hg₂Cl₂ →Δ Hg + HgCl₂
- It turns black when treated with NH₄OH.
Hg₂Cl₂ + 2NH₄OH → Hg + Hg + NH₄Cl + 2H₂O (black) - It is reduced to metallic mercury with SnCl₂.
SnCl₂ + Hg₂Cl₂ → 2Hg + SnCl₄ - It absorbs ammonia gas and forms an addition compound.
Hg₂Cl₂ + 2NH₃ → Hg₂Cl₂·2NH₃
Mercuric Chloride (HgCl₂) or Corrosive Sublimate
Preparation
(i) Hg + Cl₂ —Δ→ HgCl₂
(ii) HgO + 2HCl → HgCl₂ + H₂O
(iii) HgSO₄ + 2NaCl → HgCl₂ + Na₂SO₄
Properties
It is a white crystalline compound. It has corrosive action, highly poisonous. It sublimes on heating.
(i) On addition of KI it gives scarlet precipitate of mercuric iodide, which is dissolved in excess of KI.
HgCl₂ + 2KI → 2KCl + HgI₂ ↓ (scarlet red)
HgI₂ + 2KI → K₂HgI₄ (Nessler’s reagent)
[Note: Alkaline solution of K₂HgI₄ is known as Nessler’s reagent, used to detect the ammonium cation.]
(ii) When H₂S is passed through its solution it forms a black precipitate of mercuric sulphide.
HgCl₂ + H₂S → HgS ↓ + 2HCl (black)
(iii) It is reduced by stannous chloride.
2HgCl₂ + SnCl₂ → Hg₂Cl₂ (white) + SnCl₄
Hg₂Cl₂ + SnCl₂ → 2Hg (black) + SnCl₄
(iv) HgCl₂ when heated with Na₂CO₃ or NaOH in solution gives a yellow precipitate of mercuric oxide.
HgCl₂ + Na₂CO₃ → HgO + 2NaCl + CO₂
HgCl₂ + 2NaOH → HgO + 2NaCl + H₂O
CO-ORDINATION COMPOUNDS
Co-ordination compounds are the compounds in which the central metal atom is linked to ions or neutral molecules by co-ordinate bonds, e.g. [Cr(H2O)5Cl]2+. If the species thus formed as given above carries positive charge, it is called a complex ion.
TERMINOLOGY
The important terms used in the study of coordination compounds are mentioned below.
Central Metal Ion
It is an acceptor atom containing vacant orbitals to which a fixed number of ligands are attached via co-ordinate bonds in definite geometrical arrangement.
Ligands
It is an ion or molecule capable of donating a pair of electrons to the central atom via a donor
atom.
Unidentate ligands. Ligands with only one donor atom, e.g. NH3, Cl-, F- etc.
Bidentate ligands. Ligands with two donor atoms, e.g. ethylenediamine, C2O42- (oxalate ion) etc.
Tridentate ligands. Ligands which have three donor atoms per ligand, e.g. (dien) diethyl triamine.
Hexadentate ligands. Ligands which have six donor atoms per ligand, e.g. EDTA.
Co-ordination Sphere
Complex ion enclosed in square bracket, it behaves as a single unit.
Ionization Sphere
Part of compound present outside co-ordination sphere, e.g
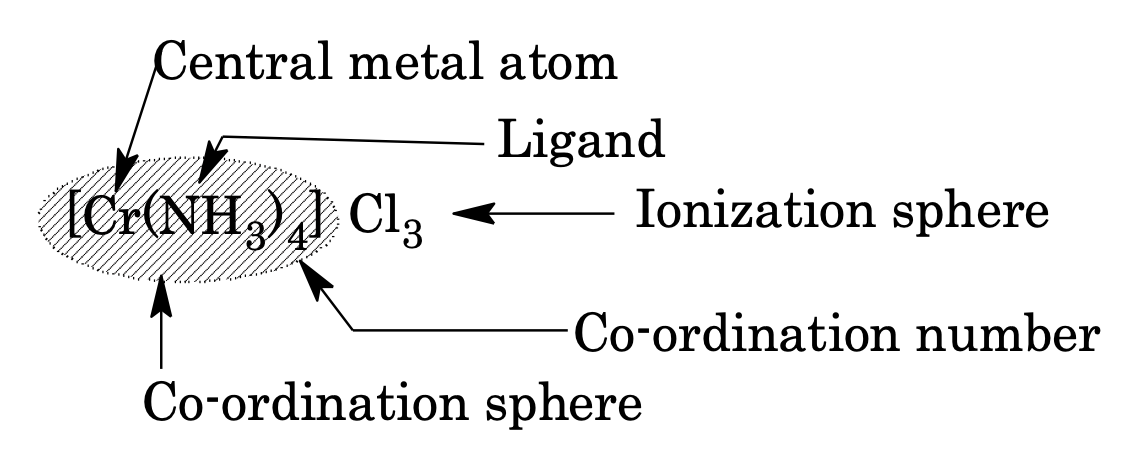
Example: The number of ions given by [Co (NH3)4]Cl3 in aqueous solution is
(A) 2
(B) 3
(C) 1
(D) 4
Solution: (D).
Co-ordination sphere does not give its ions individually of its metal atom ions.
Co-ordination Number
It is the total number of ligands attached to the central metal atom through co-ordinate bonds or the number of atoms of a ligand attached to the same central atom, e.g. hexadentate ligand should be counted as forming six co-ordinate bonds.
Oxidation number
It is the charge which the central atom appears to have if all the ligands are removed along with the electron pairs that are shared with the central atom.
[Cr(H2O)4Cl2]+
x + (4 × 0) + (−1 × 2) = +1
x + 0 – 2 = +1 = 3
[ ∵ the ligand H2O is neutral and 2 Cl− carries −2 charge ]
Chelating Ligands
Multidentate ligand simultaneously co-ordinating to a metal ion through more than one site is called chelating ligand. These ligands produce a ring like structure called chelate. Chelation increases the stability of complex. This effect is called chelation effect.
EAN (Effective Atomic Number)
It is the resultant number of electrons with the metal atom or ion after gaining electrons from the donor atoms of the ligands. EAN generally coincides with atomic number of next inert gas in some cases.
EAN = (Atomic number of metal) – (Number of e⁻ lost in ion formation) + (Number of e⁻ gained from donors)
For example, in K₄[Fe(CN)₆]
EAN = (26 – 2) + (6 × 2) = 24 + 12 = 36, i.e. atomic number of Kr.
RULES OF NOMENCLATURE
- Cations are written first followed by anions. The name starts with a small letter and is written as one word.
- Negative ligands end in –o, positive ligands end in –ium while neutral ligands have no special ending. Organic ligands are written as such in brackets.
For complicated ligands we use abbreviation, e.g. NO2+ (nitronium), NO+ (nitrosonium), CH3 (methyl), en (ethylenediamine), CH3COO− (acetate) etc. - For more than one ligand of the same type the number is indicated by di, tri, tetra. For ligands having words like ethylenediamine, the number is indicated by bis, tris, tetrakis, etc. For example, four CN are written as tetracyano while four(en) are written as tetrakis (ethylenediamine).
- For cationic complex or non ionic complex, name of metal is written as such,
e.g. [Ni(NH3)4]SO4 is tetraamminenickel (II) sulphate.
If the complex is anionic, name of metal ends in –ate followed by oxidation state,
e.g. Cr (chromate), Mn (manganate), Fe (ferrate) etc.
If the co-ordination compound is an acid, name of metal atom ends in –ic,
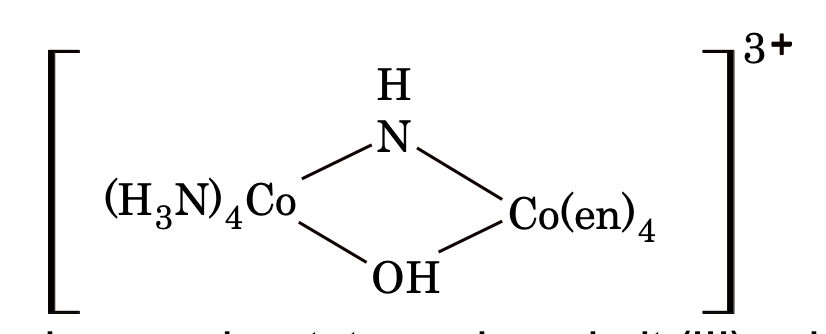
e.g. H4[PtCl6] is hexachloroplatinic (II) acid. - For polynuclear complex word -μ- is added before as name of bridging ligands, e.g.
6. For ambident ligands, point of attachment with central metal atom is either indicated by using different names for the ligand, e.g. –SCN⁻ is named as thiocyanato and –NCS⁻ is named as isothiocyanato or isothiocyanato -S- and thiocyanato -N-.
Naming optical isomers: Dextro and laevo rotatory optically active compounds are respectively designated as either by (+) and (–) or d– and ℓ–, e.g. d–K₄[PtCl₆] is potassium (+) or d-hexachloroplatinate.
Example: The IUPAC name of [Cr(H₂O)₄Cl₂]Cl is
(A) tetrahydroidichlorochromium (III) chloride
(B) tetrahydroidichlorochromium (II) chloride
(C) tetrahydroidichlorochromium (I) chloride
(D) None of the above
Solution: (B).
ISOMERISM IN CO-ORDINATION COMPOUNDS
The types of isomerism exhibited by coordination compounds are broadly divided into two types,
1. Structural isomerism
2. Stereoisomerism
Structural Isomerism
Ionization isomerism. When compounds of same molecular formula have different ions in the solution, e.g. [CoBr(NH3)5]2+ SO42– and [CoSO4(NH3)5]+ Br–.
Hydrate isomerism. When ligand H2O molecules become water of hydration outside the co-ordination sphere, e.g. [Cr(H2O)6]Cl3 is violet. Its isomer [CrCl(H2O)5]Cl2·H2O is pale green while [CrCl2(H2O)4]Cl·H2O is dark green.
Linkage isomerism. Complexes in which ambidentate ligands [e.g. –SCN– (thiocyanato) and –NCS– (isothiocyanato)] are present, show linkage isomerism, e.g. [Co(H2O)5SCN]2+ and [Co(H2O)5NCS]2+.
Co-ordination isomerism. The complexes in which both cations and anions are complexes and ligands may interchange their positions between the two complex ions, show coordination isomerism, e.g. [Cu(NH3)4][PtCl4] and [PtCl(NH3)4][CuCl4].
Co-ordination position isomerism. It arises in the bridged complexes due to the difference in the attachment of ligands with the metal atoms, e.g.
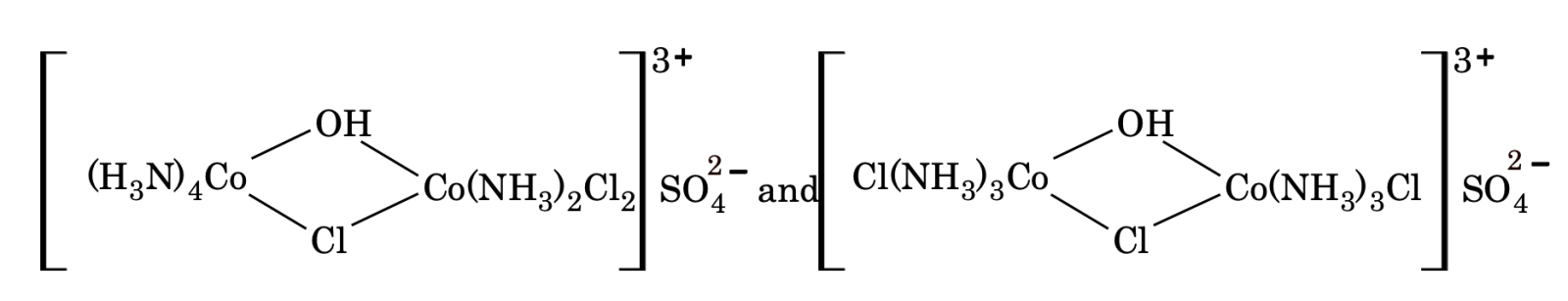
STEREOISOMERISM OR SPACE ISOMERISM
Geometrical isomerism. It is also referred as cis-trans isomerism. When same donor atoms of same ligands (groups) are on adjacent positions, it is called cis isomer while when they are on opposite positions, they are called trans isomers. Such isomerism is usually seen in complexes with co-ordination number 4 (square planar type) and 6 (octahedral).
Co-ordination number 4. Tetrahedral complexes do not show this isomerism. Square planar complexes of type MA2X2, MA2XY, MABY2, M(AB)2 and MABXY show cis-trans isomerism.
MA2X2 type [Pt(NH3)2Cl2]
MA2XY [Pt(NH3)2ClNO2]
MABXY [Pt(NH3)(H2O)(NO2)Cl]NO2
Co-ordination number 6. Octahedral complexes of type M(AA)2X2 and M(AA)2XY show cis-trans isomerism, where (AA) is symmetrical bidentate ligand. Octahedral complexes with all six ligands different (MABCDEF) exist in 15 theoretical isomers.
Optical isomerism. Chiral molecules which do not have plane of symmetry show optical isomerism. Complexes with co-ordination number 4 and 6 having chelating ligands show this kind of isomerism.
Complexes with co-ordination number 4. Square planar complexes having plane of symmetry are optically inactive. Tetrahedral complexes containing unsymmetrical bidentate ligands, i.e. M(AB)2 type show optical activity.
Complexes with co-ordination number 6. Octahedral complexes of the type [M(AA)X2Y2], [M(AA)2X2] and [M(AA)2XY] exists as pair of enantiomers. Trans forms of these complexes are symmetrical and hence optically inactive, cis isomers due to unsymmetry are optically active. Type M(AA)3 are also optically active, e.g. Cr(en)3.
Complexes having all six ligands different and complexes with hexadentate ligands such as EDTA are optically active and have d and l isomers.
Bonding in co-ordination compounds
Valence bond theory explains the shapes of complexes and relation between observed magnetic behaviours. The shapes can be explained on the basis of hybridization.
|
Shapes |
Hybridization |
|
Linear |
sp |
|
Tetrahedral |
sp3 |
|
Octahedral |
dp3/d2/d2sp3 |
|
Square planar |
dsp2 |
|
Trigonal bipyramidal |
dsp3 |
[Co(CN)6]3- has octahedral shape as explained on the basis of hybridization d2sp3.
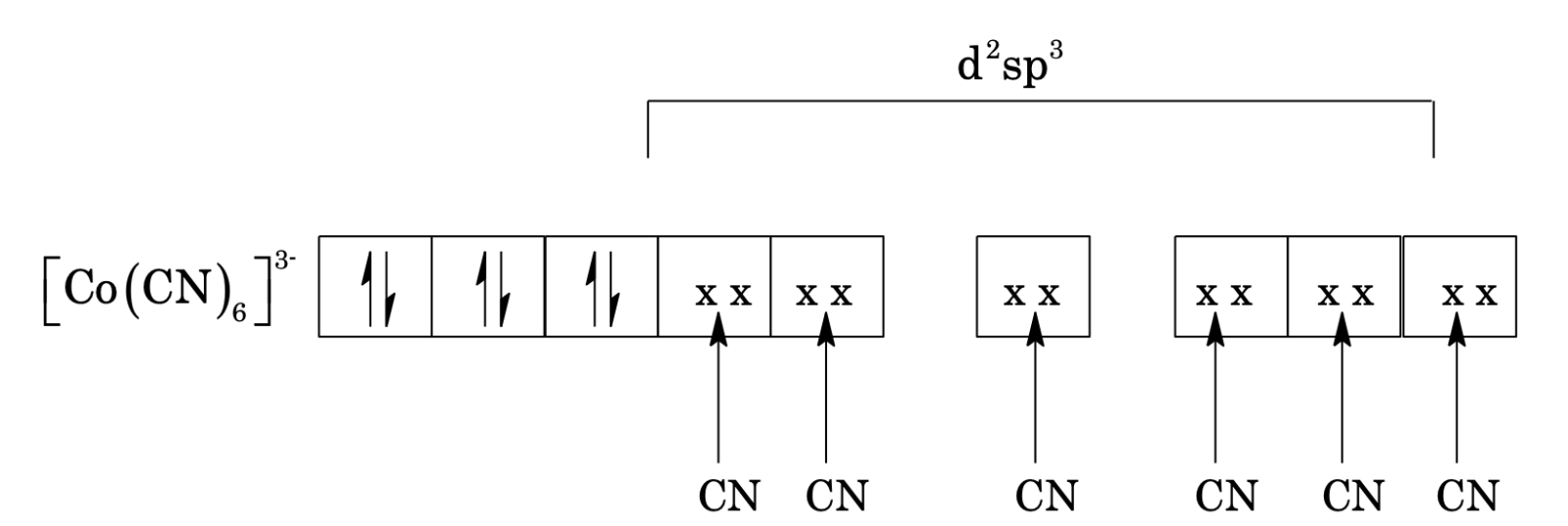
Complex is diamagnetic due to all paired electrons and absence of any unpaired electrons. Other examples:
Coordination Complexes and Their Properties
| S.No. | Complex | Metal ion | Hybridization | No. of unpaired e̅s |
|---|---|---|---|---|
| 1. | [V(H2O)6]3+ | V3+ | d2sp3 | 2 |
| 2. | [Cu(NH3)2]+ | Cu+ | sp | 0 |
| 3. | [Co(CN)6]3– | Co3+ | d2sp3 | 0 |
| 4. | [Pt(NH3)2Cl2] | Pt2+ | dsp2 | 0 |
| 5. | [CuCl4]2– | Cu2+ | sp3 | 1 |
The complexes involving (n-1)d orbitals in hybridization, i.e. dsp2 or d2sp3 hybridization are called inner d-orbital complexes. Such complexes are usually diamagnetic. Thus they are called spin paired complexes. Complexes involving outer nd orbtials in sp3d2 hybridization are called outer d orbital complexes or high spin complexes.
CRYSTAL FIELD THEORY
This theory assumes that metal ion and the ligands act as point charges and interaction between them is electrostatic. In an isolated gaseous metal atom/ion, the five d-orbitals are degenerate. But in a complex the splitting of energy of d-orbitals takes place. The splitting depends upon the nature of geometry like octahedral, tetrahedral or square planar and the basic strength of ligands.
In case of octahedral complexes, the six ligands approach the central metal atom symmetrically along the three axis. The d-orbitals of central atom which lie along the axes (i.e. dz2 and dx2–y2), called e.g. orbitals get repelled more strongly by negative ligands which raises their energy. While the inter axial (dxy, dyz and dzx orbitals) are called t2g orbitals get lowered in energy relative to average energy of d-orbitals (before splitting), as shown below:
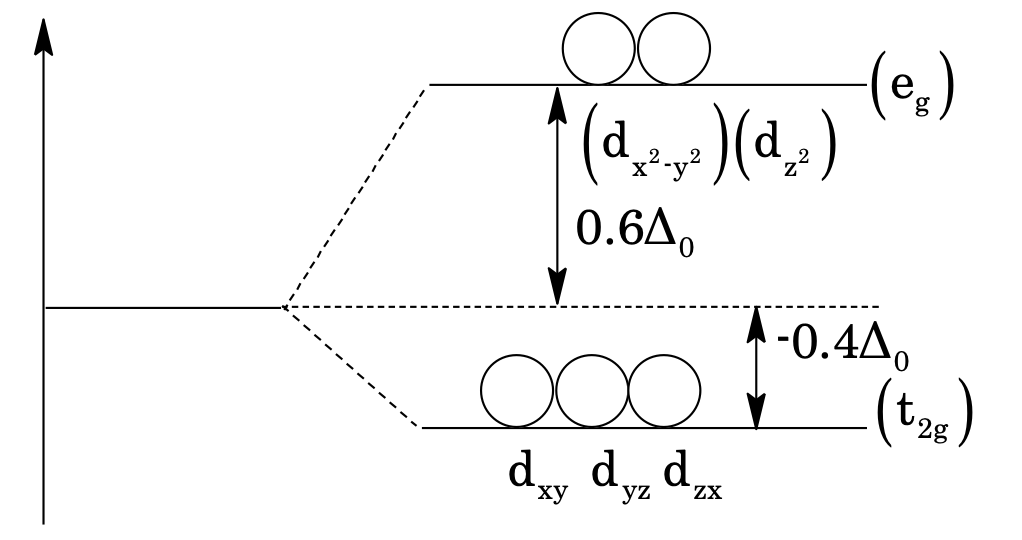
Energy difference between t₂g and eₙ set is measured in Δ₀ or Dq, where, Δ₀ = 10 Dq.
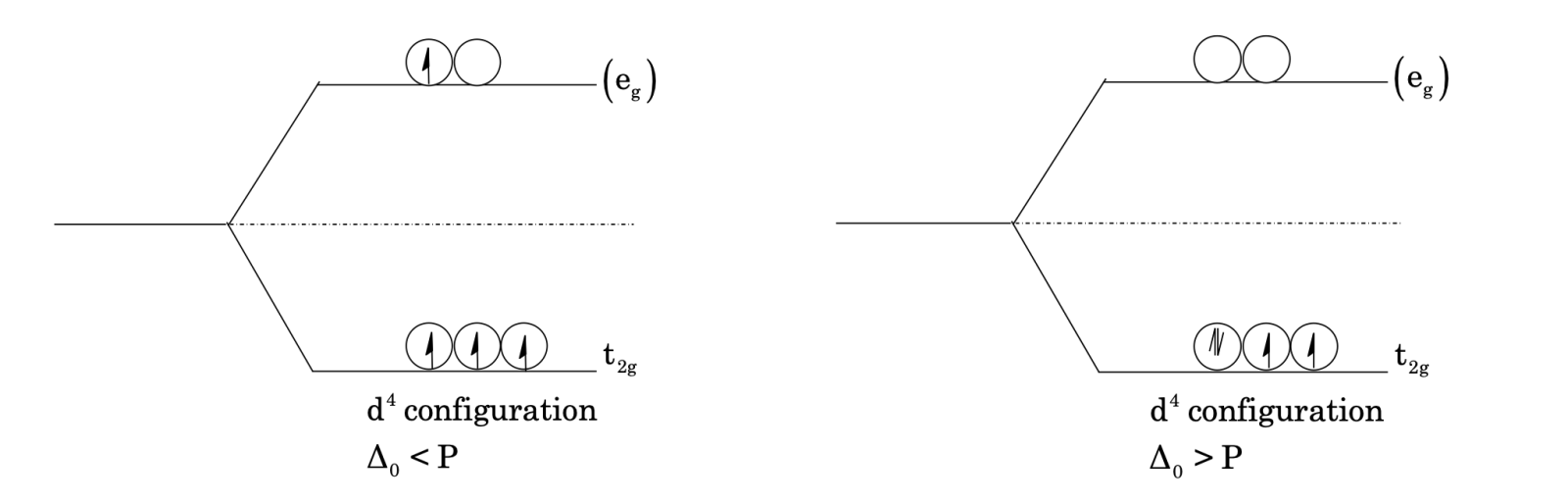
The electrons while filling occupy the lower energy set which stabilizes the system. Filling of electrons in eₙ set destabilizes it. For configuration d¹, d² and d³ lower set is filled first in accordance with Hund’s rule. For d⁴ configuration, the fourth electron goes to eₙ set, if splitting is less and is done by weak ligands like H₂O, F⁻, Cl⁻, Br⁻, I⁻. Hence, all electrons are unpaired giving high spin complexes. But for strong field ligands the splitting is much more and Δ₀ > P, so pairing takes place and fourth electron again enters t₂g set. [P = Pairing energy]
In Tetrahedral Complexes
The ligands are present between the axes due to which the inter axial orbitals experience more repulsion and have higher energy. Hence, reverse of octahedral type splitting takes place.
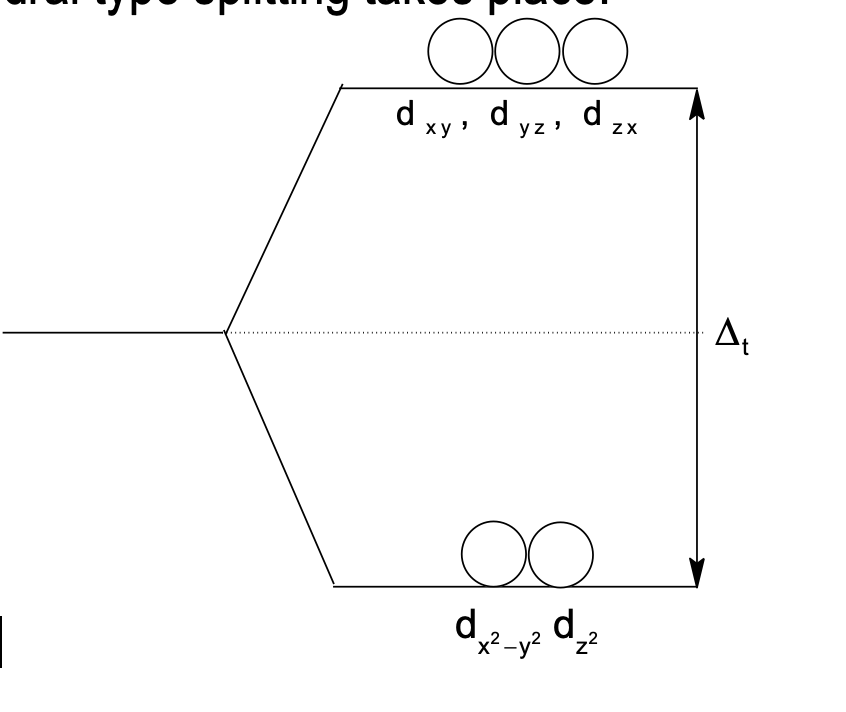
Δₜ ≈ 4⁄9 Δ₀
Tetrahedral complexes have four ligands hence splitting is less due to which only high spin tetrahedral complexes are formed.
Conditions for Stability of Complexes
- Greater the charge on central atom, greater is the splitting, greater is the stability.
- More the basic strength of ligands, more is the splitting, greater is the stability.
- More the basic strength of ligands, more is the stability.
ORGANOMETALLIC COMPOUNDS
Compounds containing at least one carbon–metal bond are called organometallic compounds.
σ Bonded Complexes
σ bonded complexes have σ bonds between carbon and metal, e.g. Pb(C₂H₅)₄, CH₃MgBr etc.
π Bonded Complexes
In π bonded complexes π electrons of ligands are involved in metal–carbon bond formation, e.g. Zeise’s salt K[PtCl₃(η²—C₂H₄)], where η² shows two carbon atoms are attached to central atom of the complex via π electron donation.
Metal Carbonyls
In metal carbonyls both σ and π bonding is involved.
A σ bond between metal and carbon is formed when vacant orbitals of metal atom overlap with an orbital on carbon atom of (CO) molecule containing a lone pair of electrons.
π bond is formed when filled orbital of metal atom overlaps with vacant antibonding. π* orbital of carbon atom of carbon monoxide. This overlap is called back donation of electrons by metal atom to carbon or π-back bonding. The give and take relationship of electrons is called synergic effect. Similarly, in olefinic complexes, the bonding π- orbital electrons are donated to empty orbitals of the metal atom and at the same time back bonding occurs from filled orbitals of the metal atom to the antibonding π-orbitals of the olefin.
CHAPTER AT A GLANCE
- All transition elements are d–block elements but all d–block elements are not transition elements. To justify this statements we take the examples of Zn. Cd and Hg which are last numbers of each d–block series. These elements are not called transition elements because they have (n − 1)d10ns2 type of completely filled electronic configuration and do not shoe the characteristic properties of transition elements except complex formation.
- First member of each transition series i.e., Sc, Y, La and Ac do not show variable valency. They show only +3 oxidation state.
3. Fe, Co and Ni are called ferrous metals; Ru, Rh, Pd, Os, Ir and Pt are called platinum metlas whereas Cu, Ag and Au are called coinage metals.
4. Cu2+ ion is more stable than Cu+ ion due to extensive hydration as it has a higher charge and lesser size as compared to Cu+ (d10 configuration) ion.
5. Both Au and Pt are inert and noble metals but they dissolve in aqua regia (3 parts conc. HCl + 1 part conc. HNO3) due to the formation of H2PtCl6 and HAuCl4 respectively.
6. TiCl4 and TiO2 are used in smoke screens; tantalum is used in surgical venals and analytical weights; chromium is used in stainless steel and chrome plating; molybdenum is used in X-rays tubes; platinum is used in resistance thermometers; zinc is used in glavanising iron sheets while cadmium is used for making joints in jewellary. TiO2 is also used as white pigment in paints, Cerium is used as a scavenger of oxygen and sulphur in many metals.
7. Finely reduce form of Pt in the form of velvety black powder is called platinum black.
8. There are many compounds of transition metals which are used as catalysts. For example,
Adam’s catalyst - Pt.PtO
Brown’s catalyst - Nickel boride
Zeigler-Natta catalyst - TiCl4 + (C2H5)3Al
Wilkinson’s catalyst - [Ph3P]3RhCl
9. There are many compound of transition elements which are used as reagents in laboratory/industry. For example,
Baeyer’s reagent - Dilute alkaline solution of KMnO4
Schweitzer reagent - [Cu(NH3)4]SO4
Nessler’s reagent - Alkaline solution of K2[HgI4]
Benedict’s solution - CuSO4 solution + sodium citrate + Na2CO3
Fenton’s reagent - FeSO4 + H2O2
Etard’s reagent - CrO2Cl2
Bordeaux mixture - CuSO4 solution + lime
Milon’s reagent - Solution of mercuric and mercurous nitrate
10. In Pt complexes 6-coordination Pt atom is generally tetravalent and 4-coordinated Pt atom is divalent. e.g., in [Pt(NH3)4][PtCl6] coordination compound, first Pt atom is divalent and second Pt atom is tetravalent.
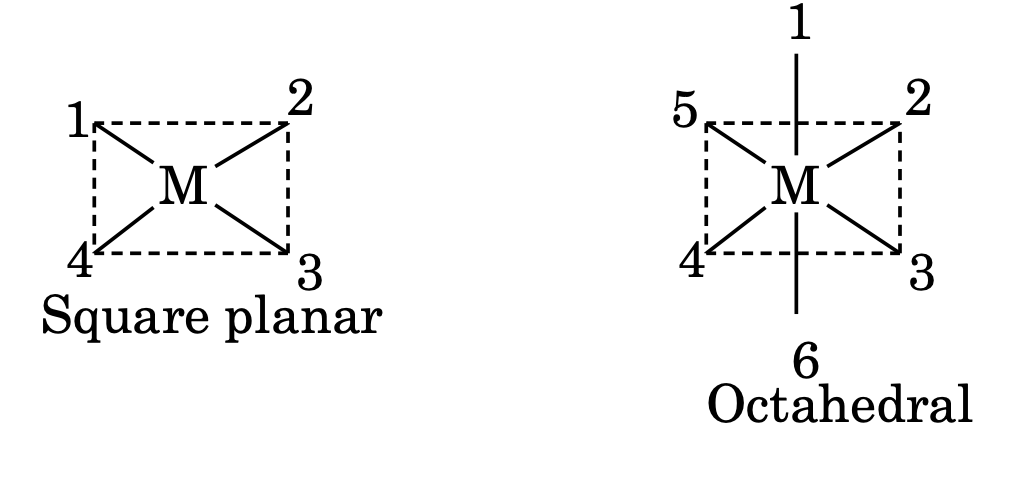
11. In square planar complexes, positions 1, 2; 2, 3; 3, 4 and 1, 4 are cis while 1, 3 and 2, 4 are trans with respect to each other. In octahedral complexes, positions 1, 2; 1, 3; 1, 4 and 1, 5 are cis while 1, 6; 2, 4 and 3, 5 are trans with respect to each other.
12. Effective atomic number (EAN) rule. Sidgwick proposed effective atomic number (EAN) to explain the stability of metal in a complex. EAN is defined as the total number of electrons on the metal atom or ion after the complex formation which should be equal to that of the next higher noble gas.
If Z is the atomic number, (O.N.) is the oxidation number and (C.N.) is the co-ordination number of the central metal atom or ion in the complex then
EAN = Z - (O.N.) + 2 ´ (C.N.)
13. Labile complex. It is that complex in which ligands can be readily replaced by other ligands.
14. Co-ordination number of the central metal atom is also called ligancy of the metal atom.
15. Prefect or penetrating complexes. These are the compounds in which complex ion is fairly stable and is either not dissociated or feebly dissociated in solution.
e.g. K₄[Fe(CN)₆] → 4K⁺ + [Fe(CN)₆]⁴⁻ ⇌ Fe²⁺ + 6CN⁻ (feebly dissociated)
16. Imperfect or normal complexes. These are the compounds in which complex ion is less stable and is reversibly dissociated to give enough simple ions.
e.g. K₂[Cd(CN)₄] → 2K⁺ + [Cd(CN)₄]²⁻ ⇌ Cd²⁺ + 4CN⁻ (appreciably dissociated)
17. The magnetic criteria of the bond type explains that the geometry of the coordination entity can be predicted if its magnetic behaviour is known. Thus, the diamagnetic [Ni(CN)₄]²⁻ is square planar while the paramagnetic [NiCl₄]²⁻ is tetrahedral.
Solved Example
1. A metal gives two chlorides A and B. A gives black precipitate with NH4OH and B gives white. With KI, B gives a red precipitate which is soluble in excess of KI. A and B are respectively
(A) HgCl2 and Hg2Cl2
(B) Hg2Cl2 and HgCl2
(C) HgCl2 and ZnCl2
(D) ZnCl2 and HgCl2
Sol. (B). Hg2Cl2 + 2NH4OH → Hg + NH2HgCl + NH4Cl + 2H2O (A) black ppt.
HgCl2 + 2NH4OH → HgNH2Cl + NH4Cl + 2H2O (B) white ppt.
HgCl2 + 2KI → HgI2 + 2KCl (B) red ppt.
HgI2 + 2KI → K2[HgI4] (soluble)
2. A blue colouration is not obtained when
(A) ammonium hydroxide dissolves in copper sulphate
(B) copper sulphate solution reacts with K4[Fe(CN)6]
(C) ferric chloride reacts with sodium ferrocyanide
(D) anhydrous CuSO4 is dissolved in water
Sol. (B).
2CuSO4 + K4[Fe(CN)6] → Cu2[Fe(CN)6] + 2K2SO4 (chocolate ppt.)
3. Which of the following ions will finally give a black precipitate with Ag⁺ ion?
(A) SO32−
(B) Br−
(C) CrO42−
(D) S2O32−
Sol. (D).
2Ag+ + S2O32− → Ag2S2O3, white ppt. which readily changes to yellow, orange, brown and finally black due to the formation of silver sulphide.
Ag2S2O3 + H2O → H2SO3 + Ag2S (black)
4. The compound which gives oxygen on moderate heating is
(A) zinc oxide
(B) mercuric oxide
(C) aluminium oxide
(D) ferric oxide
Sol. (B).
2Hg2O —heat→ 4Hg + O2
5. Which one of the following has the least magnetic moment?
(A) Cu2+
(B) Ni2+
(C) Co2+
(D) Fe2+
Sol. (A).
Cu2+ = 3d9 (1 unpaired electron); Ni2+ = 3d8 (2 unpaired electrons); Co2+ = 3d7 (3 unpaired electrons) and Fe2+ = 3d6 (4 unpaired electrons). Hence, Cu2+ shows least magnetic moment.