Alkyl halides or haloalkanes are the class of organic compounds in which a halogen is bonded to an alkyl group. The general formula of RX (where R is an alkyl group, and X is a halogen atom) is CnH2n+1, e.g. CH3Cl, C2H5Cl. Unsaturated hydrocarbons also form halogen derivatives. For example:
- CH2=CH-Cl - Chloroethene (Vinyl chloride)
- CH2=CH-CH2Cl - 3-chloroprop-1-ene (Allyl chloride)
- H3C-CH=CH-CH2Cl - 1-chlorobut-2-ene (Crotyl chloride)
- 3-chloro-1-phenylprop-1-ene (Cinnamyl chloride)
Aromatic halogen compounds or haloarenes are the halogen compounds containing at least one benzene ring. Halogen derivatives of aromatic compounds are of two types:
Aryl halides
In this type of compounds, the halogen atom is directly linked to the carbon of benzene nucleus. They are also called nuclear substitution derivatives.
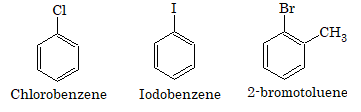
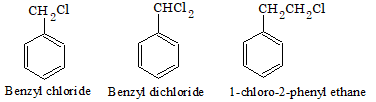
Aralkyl halides
In this type of compounds, halogen is linked to the carbon atom of the side chain attached to benzene ring. They are also called side chain substitution derivatives.
Preparation of Haloalkenes
By Direct Halogenation of Alkanes
When alkanes are treated with halogen, chlorine or bromine, in the presence of light or heat, a mixture of mono and poly substituted products is obtained.
RH + X2 → R-X + HX (UV light or heat)
CH4 + Cl2 → CH3Cl + HCl CH3Cl + Cl2 → CH2Cl2 + HCl CH2Cl2 + Cl2 → CHCl3 + HCl → CHCl3 + Cl2 → CCl4 + HCl
In the above reaction, the substitution beyond monohalogenation can be suppressed by using alkane in excess. But the method is not practical because of the difficulties arise during separation of the mixture. In case of higher alkanes, different isomeric products are formed even when monosubstitution is carried out.
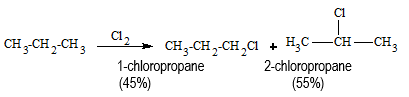
In general, the ease of substitution of different types of hydrogen atoms follows the order: benzylic > allylic > tertiary > secondary > primary > vinylic, aryl
The iodination of alkanes is reversible and is done by heating the alkane with iodine in the presence of oxidizing agents like conc. HNO3, HIO4 or HIO3. The function of using such agents is to oxidize HI, formed during the reaction, to iodine, and hence shift the equilibrium in the forward direction.
CH4 + I2 ⇌ CH3I + HI (heat)
5HI + HIO3 → 3I2 + 3H2O
By Addition of HX on Alkenes (HX = HCl, HBr, HI)
RCH=CHR + HX → RCH2-CHXR
CH2=CH2 + HX → CH3CH2X
In unsymmetrical alkenes the addition of halogen acids takes place according to the Markonikov's rule. Anti-Markonikov's addition is observed during addition of HBr in presence of peroxide (H2O2), known as peroxide effect or Kharasch effect.
From Alcohols
Action of halogen acids
Primary alcohols need ZnCl2 (anhydrous) for preparation of alkyl halides. The mixture of HCl and anhydrous ZnCl2 is known as Lucas reagent.
R-OH + HCl(g) or HCl(conc.) → RCl + H2O (ZnCl2)
ROH + HBr → RBr (48%)
Bromoalkanes can be obtained by heating alcohols with KBr or NaBr and conc. H2SO4. HBr is generated in situ.
KBr + H2SO4 → KHSO4 + HBr
C2H5OH + HBr → C2H5Br + H2O
Iodoalkanes are obtained by heating alcohols with KI and 95% H3PO4. The reactivity order of alcohols is: tertiary > secondary > primary The reactivity order of halogen is: HI > HBr > HCl
By reaction with phosphorus halides
Chloroalkanes are obtained by reaction of alcohols with PCl3 or PCl5.
3ROH + PCl3 → 3RCl + H3PO3 ROH + PCl5 → RCl + POCl3 + HCl
Note: PBr3 and PI3 being less stable, hence for bromides and iodides, P + Br2 or P + I2 mixture is used.
Action of Thionyl Chloride or Darzen's Method
R-OH + SOCl2 → RCl + SO2 + HCl (Pyridine)
Note: SOBr2 is less stable and SOI2 does not exist hence bromides and iodides are not prepared by this method.
By Halide Exchange
(Finkelstein reaction) Iodides are usually prepared by this method.
RX + NaI → RI + NaX (X = Cl, Br) (Acetone)
Fluoroalkanes (which is difficult to prepare directly by action of alkanes) can be obtained by treating alkyl halides with salts like AgF, Hg2F2, CoF3 or SbF3. This reaction is known as Swarts reaction.
2CH3CH2Cl + Hg2F2 → 2CH3CH2F + Hg2Cl2
From Silver Salts of Fatty Acids (Borodin–Hunsdiecker reaction)
Alkyl chlorides and alkyl bromides are obtained by the action of Cl2 or Br2 in CCl4 on silver salt of fatty acids. The reaction proceeds through free radical mechanism.
RCOOAg + X2 → RX + CO2 + AgX (Reflux)
Iodoalkanes cannot be obtained by this method because I2 reacts with silver salts of fatty acids to form ester.
2RCOOAg + I2 → RCOOR + CO2 + 2AgI
This reaction is called Birnbaurn–Simoni reaction.
Illustration 1:
The best method to prepare fluoroethane is
- C2H5OH + HF/H2SO4
- C2H5OH + HF/SbF5
- C2H5Cl + Hg2F2
- C2H6 + F2, hν
Solution: (C) Fluoroethane is prepared by halogen exchange method, i.e. Swarts reaction.
2C2H5Cl + Hg2F2 → 2C2H5F + Hg2Cl2
Exercise 1
Consider the following reaction sequence:
CH3C≡CH → A → B (aq. H2SO4/HgSO4, heat) → (PCl5)
The products (A) and (B) are:
- CH3COCH3 and CH3CCl2CH3
- CH3CH2CHO and CH3CH2CHCl2
- CH3CHOHCH3 and CH3CHClCH3
- CH3CH2CH2OH and CH3CH2CH2Cl
Preparation of Haloarenes
By Direct Halogenation
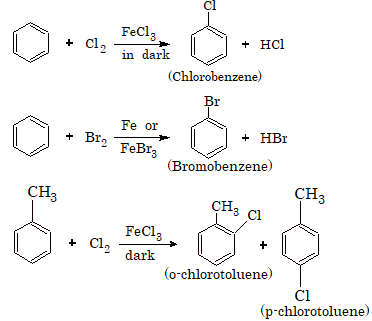
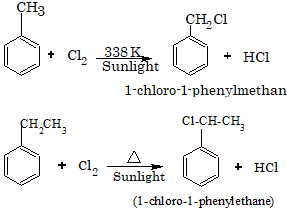
The alkyl benzene when heated with halogens in the presence of sunlight and in the absence of Lewis acids (halogen carrier) undergoes substitution at the alkyl chain resulting in the formation of aralkyl halides.
For Example
From Diazonium Compounds
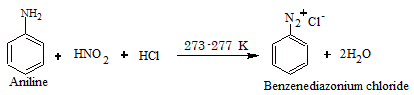
The reaction is known as diazotization.
The diazonium compound is treated with CuCl and HCl or CuBr and HBr to give the corresponding haloarene. This reaction is known as Sandmeyer reaction.
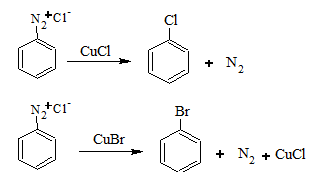
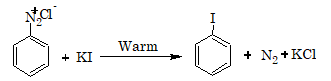
If instead of CuCl and CuBr, Cu powder and HCl or HBr is used, the reaction is called Gattermann's reaction. Iodoarenes are obtained by warming benzenediazonium salts with KI.
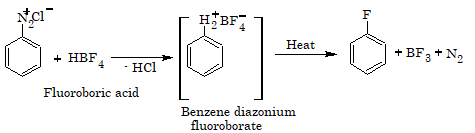
Fluoroarenes are obtained by the reaction of corresponding diazonium salts with fluoroboric acid to produce diazonium fluoroborate which on heating produces fluorobenzene. This reaction is called Balz–Schiemann reaction.
From Benzene (Commercial Method)
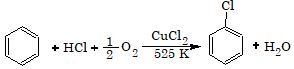
This process is known as Raschig process.
By Hunsdiecker Reaction
C6H5COOAg + X2 → C6H5X + CO2 + AgX (Cl2 or Br2, CCl4/Xylene)
Exercise 2
CH3-benzene + Br2/FeBr3 → A
Product A is:
- o-bromotoluene
- m-bromotoluene
- p-bromotoluene
- 50% o-bromotoluene + 50% p-bromotoluene
Physical Properties of Haloalkanes
- Physical state: CH3Cl, C2H5Cl, CH3Br are gases at room temperature. The alkyl halide up to C18 are liquids, while higher are colourless solids.
- Boiling point: The boiling point of haloalkanes are in the order RCl < RBr < RI. It is because with the increase in size and mass of the halogen atom, the magnitude of van der Waals forces of attraction increases. Among isomeric alkyl halides, the boiling point decreases with increase in branching in the alkyl group. This is due to the reason that with increase in branching the molecule acquire less surface area due to attainment of spherical shape. As a result, interparticle forces become weaker, resulting in lower boiling point.The boiling point of various halogen compounds increases with the increase in number of halogen atoms. The boiling point of alkyl halides shows following order:
- Alkyl iodide > Alkyl bromide > Alkyl chloride (for a given alkyl group)
- Methyl halide < Ethyl halide < Propyl halide (for a given halide)
- 1° halide > 2° halide > 3° halide (for a given halide and alkyl group)
- Solubility: The alkyl halides are polar in nature but still they are insoluble in water as they do not form H-bonds with water molecules.
Physical Properties of Haloarenes
- Boiling point: The boiling point of monohalogen derivatives of benzene, which are all liquids, follow the order: iodo > bromo > chloro. The boiling point of isomeric dihalobenzenes are nearly the same. However, their melting points are quite different.
- Solubility: They are soluble in organic solvents like alcohol and ether but insoluble in water as they cannot form H-bonding with water molecules.
- Density: These compounds are heavier than water. Their densities follow the order: Iodo > Bromo > Chloro.
Chemical Properties of Haloalkanes
The alkyl halides are highly reactive due to high electronegativity difference between carbon and halogen atom, which provides polarity in C-X bond. Thus, carbon atom of C-X bond is easily attacked by a nucleophile and shows nucleophilic substitution.
R-X + :Nu- → R-Nu + X-
The nucleophilic substitution may follow SN1 or SN2 mechanism. In addition to nucleophilic substitution alkyl halides also show elimination reactions.
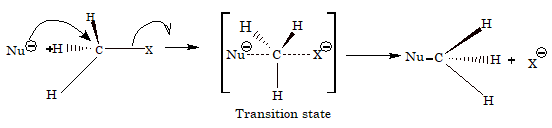
SN2 mechanism
The rate of reaction is dependent on the concentration of alkyl halide as well as the nucleophile, i.e. Rate = K[RX][Nu-]. Hence, the reaction is bimolecular nucleophilic substitution. There is complete inversion of configuration as it involves attack of nucleophile from backside.
SN1 Mechanism
The rate of reaction is dependent only on the concentration of alkyl halide, i.e. Rate = K[RX]. Hence, the reaction is unimolecular nucleophilic substitution. The mechanism involves two steps:
Step 1: In the first step, the alkyl halide slowly dissociates into halide ion and carbocation.
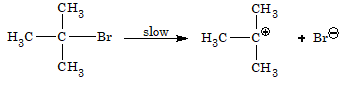
Step 2: In the second step, carbocation formed immediately combines with the nucleophile to form the final substituted product.
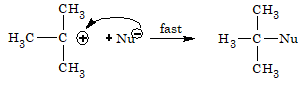
The order of reactivity of various alkyl halides towards nucleophilic substitution reaction by SN1 mechanism is: 3° > 2° > 1°
A racemised product is obtained by SN1 mechanism. This is due to the fact that the carbocation formed has planar structure. So, the nucleophile can attack this carbocation from both sides.
Nucleophilic Substitution on Alkyl Halides
The halogen atom of alkyl halide is easily replaced by a nucleophile to give a large variety of nucleophilic substitution reactions.
Replacement by hydroxyl group (formation of alcohols):
R-X + HOH (boil) → R-OH + X-
R-X + KOH(aq.) → R-OH + KBr
R-X + Ag2O(moist) → R-OH + AgBr
Replacement by alkoxy group (formation of ethers): In this reaction, alkyl halide is treated with alcoholic sodium or potassium alkoxide to form ether. This reaction is known as Williamson's synthesis.
RX + Na-OR' → R-O-R' + X-
C2H5Br + NaOC2H5 → C2H5OC2H5 + NaBr (Bromoethane → Diethyl ether)
Replacement by cyano group (formation of cyanides or nitriles):
RX + KCN(alc.) → R-C≡N + X- + RNC (Alkane nitrile - major)
CH3CH2I + KCN(alc.) → CH3CH2C≡N + KI (Iodoethane → Propane nitrile)
Replacement with Isocyanide (formation of isocyanide):
RX + AgCN(alc.) → R-N≡C + AgX + RCN (major)
C2H5Br + AgCN(alc.) → C2H5-N≡C + AgBr (Ethyl isocyanide - major)
Replacement by amino group (formation of amines):This reaction is known as Hofmann's ammonolysis.
RX + NH3(alc.) → R-NH2 + HX
If haloalkane is present in excess, then other two hydrogen atoms of amino group are also replaced by alkyl groups leading to the formation of secondary and tertiary amines. Tertiary amine so formed further combines with alkyl halide to give quaternary ammonium salt.
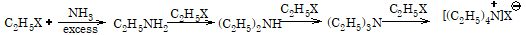
Replacement by nitro group
Treatment of alkyl halide with AgNO2 gives nitro alkane as a major product.
R-X + AgNO2 → R-NO2 + AgX
Replacement with nitrite group
Treatment of alkyl halide with potassium nitrite gives alkyl nitrite as a major product.
R-X + KNO2 → R-O-N=O + KX
Replacement with -SH (hydrosulphide) group
Alkyl halides on reaction with hydrosulphide group forms thiols or mercaptans.
R-X + NaSH → R-SH + NaX
Replacement by mercaptide (SR-)
Alkyl halides on reaction with mercaptide ion gives thio ethers.
R-X + R'S-Na+ → R-S-R' + NaX
Thioethers can also be prepared by reaction of alkyl halides with sodium or potassium sulphide.
2R-X + Na2S → R-S-R + 2NaX
Replacement by alkynyl group
Higher alkynes can be prepared on reacting alkyl halides with alkynyl group.
R-C≡C-Na+ + R'-X → R-C≡C-R' + NaX
Note: The reaction is of limited practical use and can be used only in case of primary halides because the secondary and tertiary halides undergo elimination rather than substitution.
Replacement by carboxylate group
Alkyl halides on reaction with silver salts of carboxylic acid gives esters.
R-X + R'COOAg → R'COOR + AgX
Replacement by hydride ion
Alkanes can be prepared from alkyl halides by this substitution reaction.
R-X + H- → R-H + X-
Dehydrohalogenation Reactions
When haloalkanes are heated with alcoholic KOH, they undergo dehydrohalogenation to form alkenes. This is also known as β-elimination reaction.
R-CH2-CH2-X + KOH (alc.) → R-CH=CH2 + KX + H2O
The reactivity of haloalkanes towards elimination reaction follows the order:
Tertiary > Secondary > Primary
Haloalkanes undergo elimination in two different ways to produce two different alkenes. The preferred alkene is one in which more number of alkyl groups are attached to the double bonded carbon atoms. This generalization is known as Saytzeff rule.
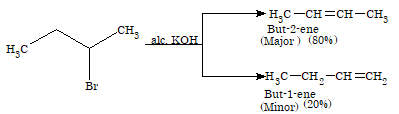
Illustration 2: Arrange the following halides in the decreasing order of SN1 reactivity
CH3CH2CH2Cl CH2=CHCH(Cl)CH3 CH3CH2CH(Cl)CH3
I II III
(A) I > II > III
(B) II > I > III
(C) II > III > I
(D) III > II > I
Solution: (C) Allyl carbocation is more stable than 2° carbocation which in turn is more stable than primary.
Illustration 3: During debromination of meso-dibromobutane, the major compound formed is
(A) n-butane
(B) 1-butene
(C) cis 2-butene
(D) trans 2-butene
Solution: (D) Debromination is a trans elimination reaction and hence meso-dibromobutane on debromination gives trans 2-butene.
Exercise 3
For the reaction, R-Br → R-O-N=O, the suitable reagent is
(A) NaNO2 + HCl
(B) HNO2
(C) AgNO2
(D) KNO2
Reactions with Metals
1. Reaction with sodium (Wurtz Reaction)
This reaction is known as Wurtz reaction and generally used to prepare symmetrical alkanes with more number of carbon atoms.
2R-X + 2Na → R-R + 2NaX
2. Reaction with magnesium
R-X + Mg → R-Mg-X (Grignard Reagent)
3. Reaction with zinc powder
2R-X + Zn → R-R + ZnX2
4. Reaction with lithium
R-X + 2Li → R-Li + LiX
5. Reaction with sodium amalgam
R-X + Na(Hg) → R-H + NaX + Hg
6. Reaction with Pb-Na alloy
4R-X + 4Pb-Na → 4R-H + 4NaX + 4Pb
Reduction
1. Reaction with H2/Ni
R-X + H2 → R-H + HX
2. Reaction with zinc-copper couple (in presence of alcohol)
R-X + Zn-Cu + C2H5OH → R-H + Products
Similarly, Zn/HCl, Zn/NaOH, Na/C2H5OH, Sn/HCl or HI/red P at 430 K can also be used to reduce haloalkanes to alkanes.
Action of Heat
Alkenes can be prepared by heating alkyl halides at a very high temperature.
R-CH2-CH2-X → R-CH=CH2 + HX
Illustration 4: The end product of the following reaction is
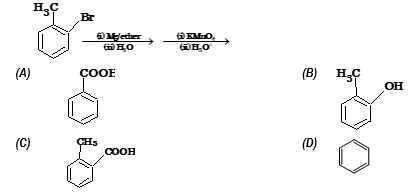
Solution: (A)
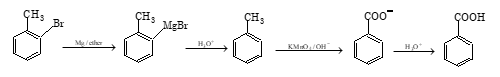
Chemical Properties of Aryl Halides
Aryl halides are much less reactive towards nucleophilic substitution reaction than haloalkanes. However, they can be made to react under drastic conditions, such as high pressure and high temperature.
Nucleophilic Substitution Reactions
1. Replacement by hydroxy group - Dow's process
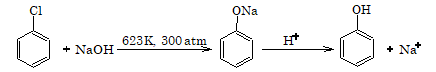
The reactivity of aryl halides towards nucleophilic substitution increases if some electron withdrawing group such as nitro, cyano or carbonyl group is attached to the ring.
2. Replacement by cyano group
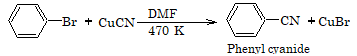
3. Replacement by amino group
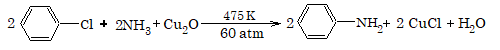
4. Replacement by alkoxide group

5. Replacement by -SH group
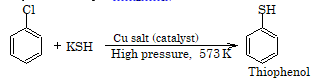
Reduction
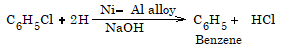
Reaction With Metals
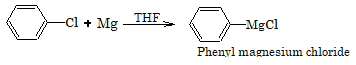
1. Reaction with magnesium
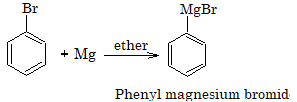
Aryl chlorides do not form Grignard reagent in presence of ether as solvent but it is possible if THF (Tetrahydrofuran) is used.
2. Reaction with sodium
Aryl halides react with sodium in presence of ether.
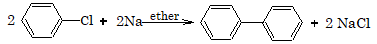
When aryl halides are treated with haloalkane and sodium in presence of dry ether, undergo Wurtz Fittig reaction.
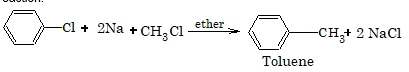
3. Reaction with lithium
Aryl halides reacts with lithium in presence of dry ether to form organometallic compound.
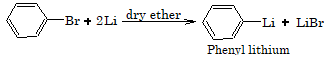
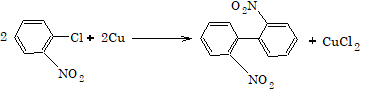
4. Reaction with copper powder (Ullmann Reaction)
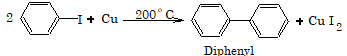
Aryl chloride and aryl bromide usually do not give this reaction, however if o and p-nitro substituted aryl chloride and aryl bromide are used, then it shows Ullmann reaction.
Ring Substitution Reactions
Aryl Halides undergo electrophilic substitution reaction in the benzene ring. The halogen atoms are o and p-directing groups but deactivates the ring at the same time. Therefore, substitution occurs relatively at slower rate in comparison to benzene.
1. Halogenation
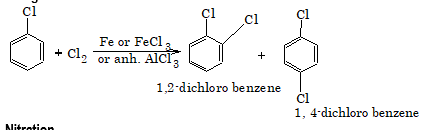
2. Nitration
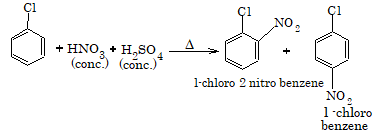
3. Sulphonation
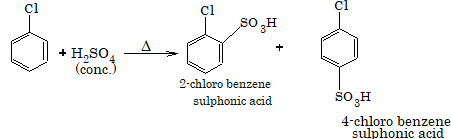
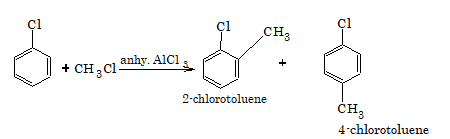
4. Friedel-Crafts alkylation
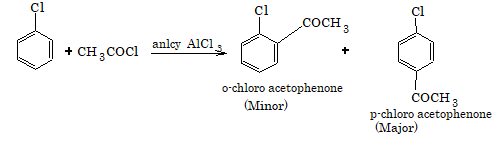
5. Friedel-Crafts acylation
6. Action with chloral
Two molecules of chlorobenzene reacts with chloral to form DDT.
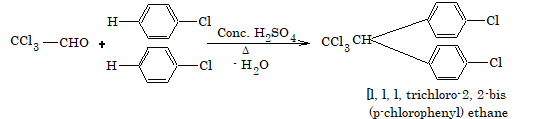
Illustration 5: Which of the following halogen containing compound will not undergo SN reactions easily?

Solution: (A) Vinyl halide cannot be substituted by other nucleophile due to double bond character and instability of vinyl cation.
Trichloromethane (Chloroform)
Methods of Preparation
1. From methane
Chloroform is prepared by chlorination of methane in the presence of light.
CH4 + Cl2 → CH3Cl + CH2Cl2 + CHCl3 + CCl4
The mixture of CH3Cl, CH2Cl2, CHCl3, and CCl4 are separated by fractional distillation.
2. From chloral hydrate
Chloroform (in pure form) can be prepared by distillation of chloral or chloral hydrate with concentrated aqueous NaOH solution or KOH solution.
CCl3CH(OH)2 + NaOH → CHCl3 + HCOONa + H2O
3. From ethanol or acetone (lab method)
Chloroform is obtained from ethanol or acetone by reaction with a paste of bleaching powder and water.
(a) In case of alcohol:
C2H5OH + Cl2 → CH3CHO + 2HCl
CH3CHO + 3Cl2 → CCl3CHO + 3HCl
2CCl3CHO + Ca(OH)2 → 2CHCl3 + (HCOO)2Ca
(b) In case of acetone:
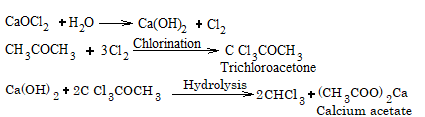
4. From carbon tetrachloride
By partial reduction of carbon tetrachloride with iron filings and water.
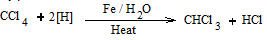
Physical Properties
Chloroform is a colourless, heavy liquid. It has sweetish, sickly odour and taste. It is heavier than water and slightly soluble in water. Its boiling point is 334 K. Inhaling of chloroform causes unconsciousness and thus used as anaesthetic.
Chemical Properties
- Action of sunlight and air: It is oxidized by air to produce a highly poisonous compound called phosgene (COCl2).
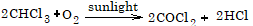
To avoid the formation of phosgene, chloroform is kept in dark bottles to cut off active light radiations and filled upto brim to keep out air. Also a little amount of 1% ethanol is added which converts the toxic COCl2 as non-poisonous diethyl carbonate.
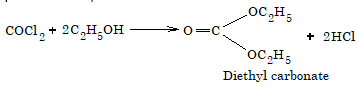
- Hydrolysis: When boiled with aqueous KOH, chloroform is hydrolysed to potassium formate.
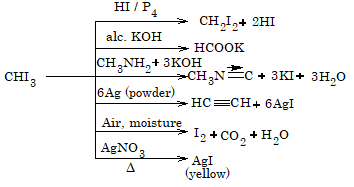
CHCL3 + 4KOH → HCOOK = 3KCl + 2H2O
- Action of Ag powder
2CHCl3 + 6Ag → HC = CH + 6AgCl
- Reaction with nitric acid
CHCl3 + HNO3 → CCl3 NO2 + H2O
Chloropicrin is used as an insecticide and war gas.
- Reaction with acetone
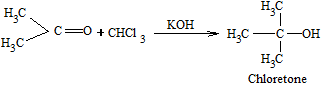
Chloretone is used as hypnotic (a sleep inducing drug).
- Reaction with primary amines (Carbylamine reaction).
RNH2 + CHCl3 + 3KOH → RNC + 3KCl + 3H2O
Both aromatic and aliphatic primary amines give isocyanide with KOH (alcoholic) and chloroform. The isocyanide formed has a characteristic smell and this reaction is known as isocyanide or carbylamine reaction. It is used for the detection of primary amines.
- Reduction with zinc and HCl
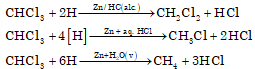
- Reaction with sodium ethoxide
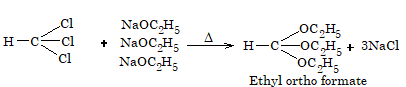
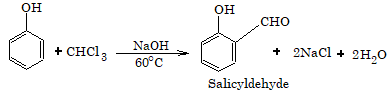
- Riemer–Tiemann reaction
- Reaction with silver nitrate: Chloroform does not give a precipitate when treated with aqueous AgNO3. It is because the C–Cl bond in chloroform is covalent and does not ionize in aqueous solution to produce chloride ions.
Triiodomethane (Iodoform)
Methods of Preparation
Iodoform is prepared by the action of iodine and alkali on acetaldehyde, methyl ketones and alcohols (which produces unit on oxidation).
- From alcohol
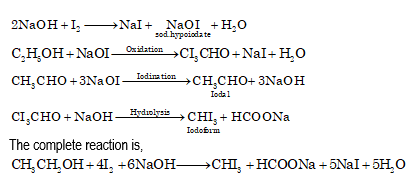
- From acetone
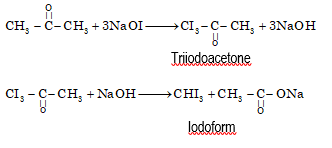
Physical Properties
Iodoform is a yellow coloured, solid compound having melting point 382 K. It is insoluble in water but dissolves readily in organic solvents.
Chemical Properties
Chemical reactions of iodoform is similar to that of chloroform.