Stuck on Chemical Kinetics Numericals? Go from 0 to Full Marks.
CBSE Chemical Kinetics notes can be personified as an "Overwhelmed Achiever." This student, typically in Class 12, is not struggling with the subject due to a lack of effort but from a specific, critical gap between theoretical knowledge and practical application. They can follow derivations of complex formulas like the Arrhenius equation, k=Ae−Ea/RT, in a classroom setting but feel paralyzed when faced with unseen numerical problems in an exam. This disconnect creates significant anxiety and a fear of underperforming in the high-stakes CBSE board exams, which they see as a crucial gateway to prestigious universities. Their primary pain point is not a lack of resources but the overwhelming nature of them, leading to frustration and a loss of confidence despite long hours of study.
Stop wasting time on confusing books. Get the only CBSE-focused notes with step-by-step methods to solve any question and ace your board exams. 100% aligned with the latest syllabus.
Trusted by 15,000+ CBSE Students from India's Top Schools
-
FORGET MEMORIZING: Our notes explain the 'why' behind every formula so you actually understand it.
-
MASTER NUMERICALS: Get proven, step-by-step guides for every single type of problem.
-
REVISE IN MINUTES: Use our one-page mind maps and formula sheets to revise the entire chapter before any test.
-
SCORE WITH CERTAINTY: Practice with a handpicked bank of the most important Past Year Questions (PYQs).
Introduction
Chemical kinetics is the branch of chemistry which deals with the rates of chemical reactions, factors affecting the rate of reaction and the mechanism of the reaction.
For any reaction, aA + bB → cC + dD the rate is given by
Rate = - (1/a) Δ[A]/Δt = - (1/b) Δ[B]/Δt = (1/c) Δ[C]/Δt = (1/d) Δ[D]/Δt
Here, minus sign indicates the decrease in concentration of reactants.
Rate of disappearance or appearance of any substance does not involve stoichiometric coefficients.
Hence, rate of disappearance of A is
- Δ[A]/Δt
e.g.
N2 + 3H2 → 2NH3
Rate = -Δ[N2]/Δt = -Δ[H2]/3Δt = Δ[NH3]/2Δt
Average rate
Average rate = (Change in concentration)/(Time interval) = (x2 - x1)/(t2 - t1) = Δx/Δt. The time interval is large here.
Instantaneous rate
Instantaneous rate is the rate of a reaction at a particular instant of time and is equal to the slope of tangent drawn at that point of time in the plot of concentration vs time.
Units of rate of a reaction are mol L–1 time–1 or atm time–1 (for gaseous reaction).
THE RATE OF A REACTION DEPENDS ON THE FOLLOWING FACTORS
(i) Concentration of the reactant: Greater the concentration of the reactants, faster is the rate.
(ii) Temperature: Generally, the rate of reaction doubles for every 10K rise in temperature.
(iii) Catalyst: It generally increases the rate by decreasing the activation energy of reaction.
(iv) Sun light: Photochemical reactions require the presence of light.
(v) Surface area of the reactants: Greater the surface area, faster is the reaction. That is why powdered coal burns faster than dump of coal.
LAW OF MASS ACTION
It states that the rate of a reaction is directly proportional to the product of active masses of the reactants each raised to the power equals to its stoichiometric coefficients.
RATE LAW
It states that the rate of a reaction is directly proportional to the product of active masses of the reactants raised to the power by the number of moles of reactants used in slowest step.
For a reaction, aA + bB → cC + dD
Rate law is given as Rate = k [A]α [B]β, where α, β are the number of moles of reactants involved in slowest step.
CHARACTERISTICS OF k
k is constant for a particular reaction, it depends upon temperature but not on the concentration of reactants.
Units of rate constant for nth order reaction is given by
1 / [conc]n−1 × 1 / time
Hence, for first order reaction the units of k is time−1.
ORDER OF A REACTION
It is the sum of powers of different combining compounds in the rate law expression. For the above reaction order = α + β.
Order is an experimental concept and it may be zero, fraction or integer.
Rate constant (k) is the rate of reaction when the concentration of each reactant is unity.
MOLECULARITY
Molecularity of a reaction is the number of atoms or ions or molecules colliding simultaneously so as to result into a chemical reaction. It is always a whole number. Molecularity of a complex reaction has no significance as such. It is the molecularity of the slowest step (rate determining step) which has significance. Hence the overall molecularity can be taken as the molecularity of the slowest step.Note: Reactions having order or molecularity more than 3 are rare. Dissociation of chlorate (4 KClO3 → 3 KClO4 + KCl) is an example of 4th order reaction. Order of a reaction changes with conditions like temperature, pressure etc. but molecularity does not.
Example: The rate of reaction between A and B increases by a factor of 100, when the concentration of A is changed from 0.1 mol L−1 to 1 mol L−1. The order of reaction with respect to A is
(A) 10
(B) 1
(C) 3
(D) 2
Solution: (D).
Change of 10 times in conc. results in changing the rate by 102 times. Hence order = 2.
MECHANISM
Mechanism of a reaction is the series of steps of overall reaction. For the reaction, 2NO2 + F2 → 2NO2F; Rate = k[NO2][F2] Hence, the proposed mechanism is NO2 + F2 slow → NO2F + F NO2 + F fast → NO2F Hence, the rate law depends on the slowest step of the mechanism only.
DIFFERENT TYPES OF REACTIONS
(i) Parallel or concurrent reactions: In such reactions reactant is converted parallely into different products.
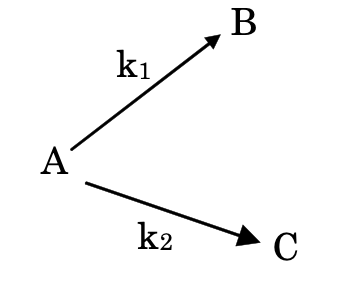
-d[A]dt = k1[A] + k2[A]
d[B]dt = k1[A]
d[C]dt = k2[A]
Hence, ratio of the two products, [B][C] = k1k2
Percentage of B = k1k1 + k2 × 100
Percentage of C = k2k1 + k2 × 100
(ii) Sequential reactions:
A —k1→ B —k2→ C
d[A]/dt = -k1[A]
d[B]/dt = k1[A] − k2[B]
d[C]/dt = k2[B]
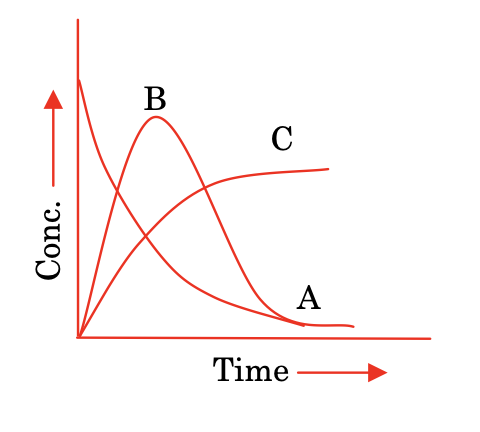
As the steady state of B is reached, the concentration of B becomes constant. Hence, rate of formation and rate of consumption of B are equal.
(iii) Reactions involving intermediate in equilibrium with reactants.
A + B k₁ ⇌ k₂ X →k₃ C
‘X’ is intermediate
Rate = k₃ [X] (i)
Keq. = k₁/k₂ = [X]/([A][B])
Hence, [X] = (k₁/k₂) [A][B]
Putting in equation (i),
∴ Rate of reaction = (k₁ k₃ / k₂) [A][B]
Reactions at equilibrium
e.g. I₂ + H₂ k₁ ⇌ k₂ 2HI
Rate of reaction = ½ d[HI]/dt = k₁[H₂][I₂] − k₂[HI]²
INTEGRATED RATE EXPRESSIONS
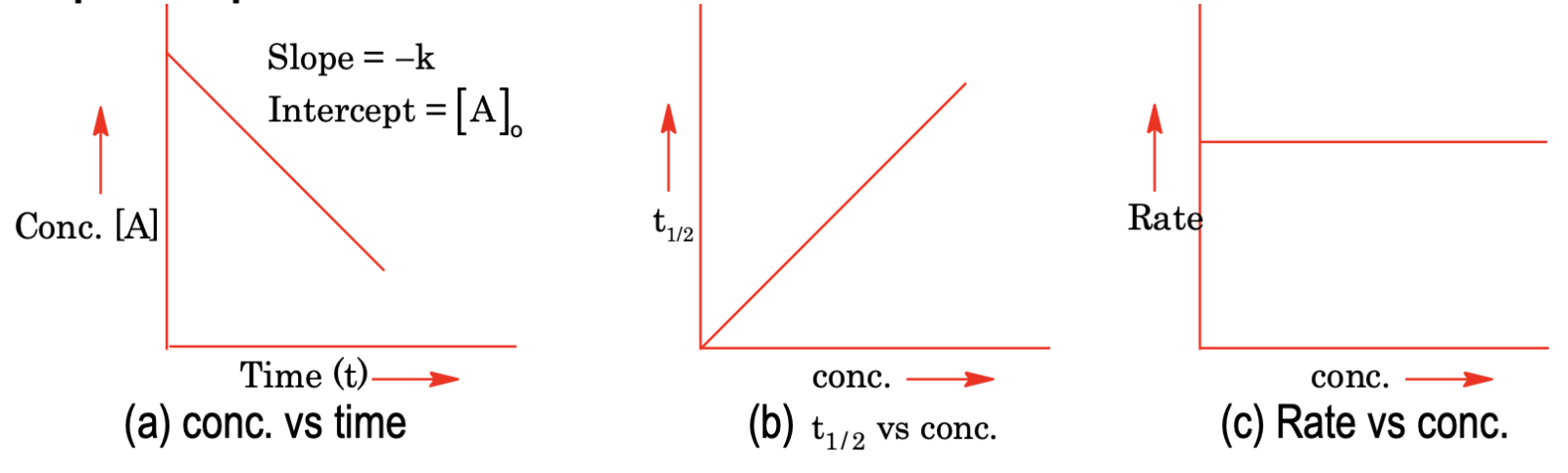
(i) Zero order reaction
For a zero order reaction, A → Product
Rate = – d[A]/dt = k[A]0 = k
On integrating, we get [A] = –kt + [A]0
or, k = (1/t) [[A]0 – [A]]
where [A]0 – initial concentration of reactant
[A] – concentration present at time ‘t’
Half life: It is the time when reaction is 50% complete.
Hence, at t1/2, [A] = [A]0 / 2
t = (1/k) [ [A]0 – ([A]0 / 2) ] = [A]0 / 2k
When whole of the substance has reacted, i.e., [A] = 0
t = [A]0 / k
Graphical representations of zero order reaction
Examples of zero order reaction
-
H2 + Cl2 ——hv——> 2HCl
- 2NH3 ——Mo or hv——> N2 + 3H2
(ii) First order reactions
For a first order reaction, A → Product
Rate = -dA/dt = k[A]
On integrating, we get
k = (2.303 / t) log([A]0 / [A]) or k = (2.303 / t) log(a / (a - x))
where [A]0 or a is the initial concentration of reactant. [A] is the present concentration and x is the amount reacted.
Exponential form for first order reaction is [A] = [A]0 e−kt.
Half life: Half life for a first order reaction is independent of the initial concentration and is given by
t1/2 = 0.693 / k
Graphical representations of first order reaction
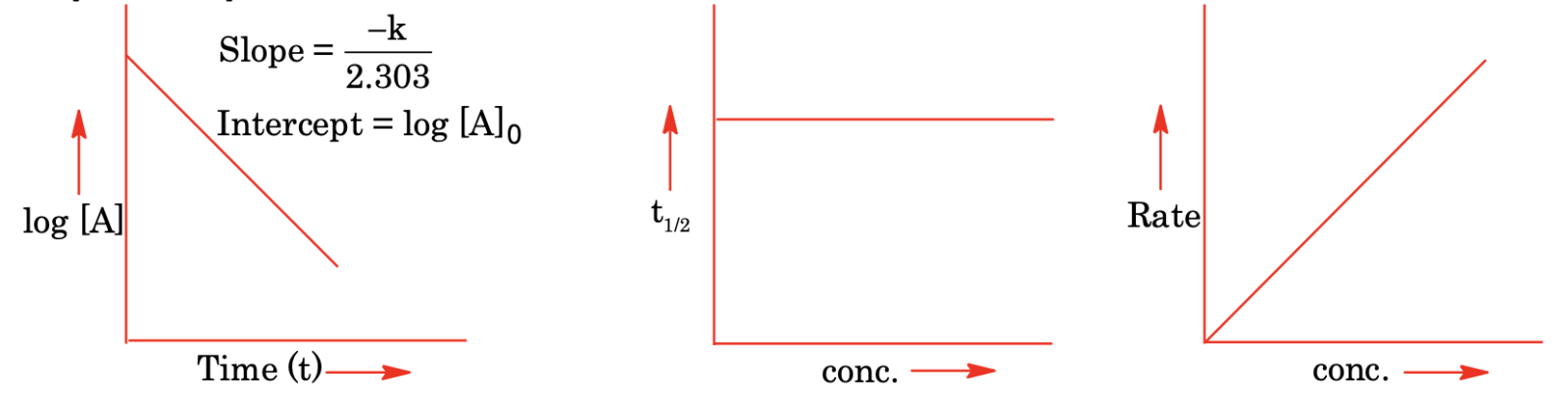
The amount of reactant left after ‘n’ half lives is given by
[A] = [A]0 / 2n
where n = Number of half lives =
Total time (t) / Half life (t1/2)
Modified expressions for rate constants of some common reactions of 1st order
(a) N2O5 → 2NO2 + ½O2
k = (2.303 / t) × log(V∞ / (V∞ - Vt))
(Vt = Volume of O2 after time t and V∞ = Volume of O2 after infinite time)
(b) NH4NO2(aq.) → 2H2O + N2
Same as above (Vt and V∞ are the volumes of N2 after time t and infinite time respectively)
(c) H2O2 → H2O + ½O2
k = (2.303 / t) × log(V0 / Vt)
(V0 and Vt are the volumes of KMnO4 solution used for titration of same volume of reaction mixture at zero time (initially) and after time t).
(d) CH3COOC2H5 + H2O →H+ CH3COOH + C2H5OH
k = 2.303⁄t log [ (V∞ − V0) ⁄ (V∞ − Vt) ]
(V0, Vt, and V∞ are the volumes of standard NaOH solution used for titration of same volume of the reaction mixture after times 0, t and ∞ respectively).
(e) Inversion of cane sugar:
C12H22O11 + H2O → C6H12O6 + C6H12O6
sucrose(d−) Glucose(d+) Fructose(ℓ−)
k = 2.303⁄t log [ (r0 − r∞) ⁄ (rt − r∞) ]
(r0, rt and r∞ are the polarimetric reading after times 0, t and ∞ respectively).
(iii) Second order reactions
For a second order reaction, A → Product
d[A]/dt = −k[A]2 or dx/dt = k(a − x)2
k = (1/t) [ x / a(a − x) ] or 1/(a − x) = kt + 1/a
t1/2 = 1/ka or 1 / k [A0]
Half life: Half life for nth order reaction is given by
t1/2 = [2n−1 − 1] ⁄ [(n − 1) k [A]0n − 1]
or t1/2 ∝ 1 / [A]0n−1, (n ≠ 1)
Graphical representations of second order reactions
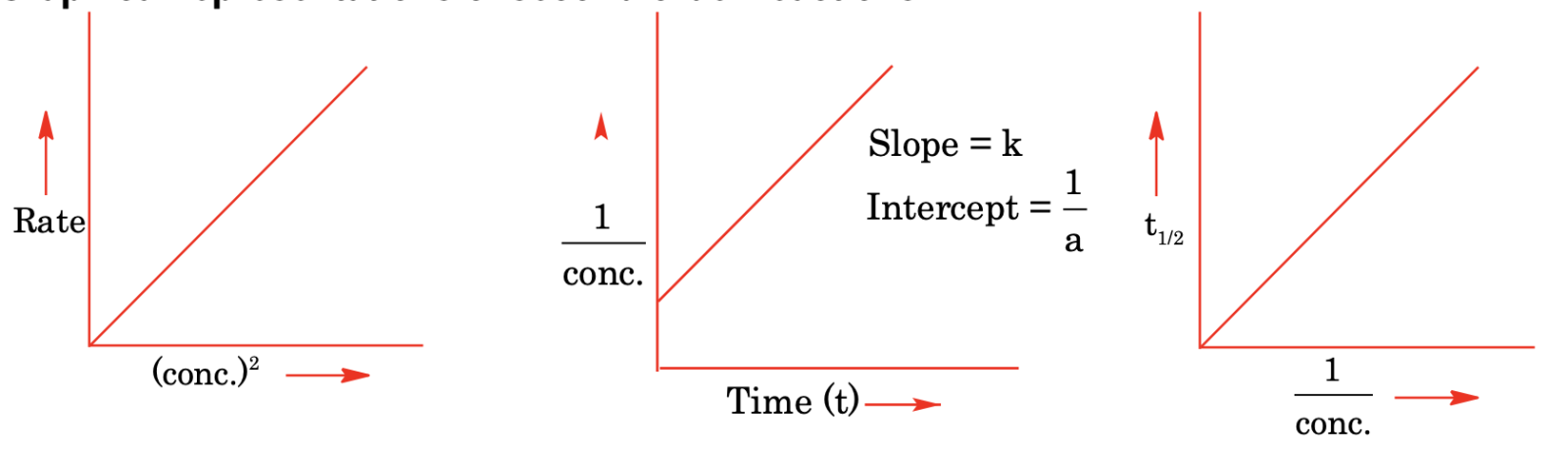
Examples of second order reaction
- (a) 2NO2 + F2 ⟶ 2NO2F
- (b) 2HI ⟶ H2 + I2
- (c) CH3COOC2H5NaOH ⟶ CH3COONa + C2H5OH
Examples of third order reaction
- (a) 2NO + O2 ⟶ 2NO2
- (b) 2NO + 2Br2 ⟶ 2NOBr
Fractional order
- CH3CHO ⟶ CH4 + CO; Rate = k[CH3CHO]3/2
- H2 + Br2 ⟶ 2HBr; Rate = k[H2][Br2]1/2
Pseudo unimolecular reactions
These reactions are not truly of first order but become first order under some specific conditions, e.g. hydrolysis of ester in acid medium, hydrolysis of cane sugar etc.
Example: Time required to decompose half of the substance for nth order reaction is inversely proportional to
(A) an+1
(B) an-1
(C) an-2
(D) a1-n
Solution: (B).
t1/2 ∝ 1 / an-1
COLLISION THEORY
Rate of reaction does not depend upon the total number of collisions but on the number of effective collisions (fraction of molecules having energy greater than threshold or activation energy).
Rate = −dx/dt = Z × f
where Z = Collision frequency
f = e−Ea/RT is the number of effective collisions
Temperature coefficient
Temperature coefficient (μ) = k308 / k298, μ lies between 2 and 3.
Generally rate of reactions doubles for every 10°C rise in temperature. If the temperature of the reaction having temperature coefficient μ is increased from T1 to T2, rate is increased by μn times where n = (T2 − T1)/10.
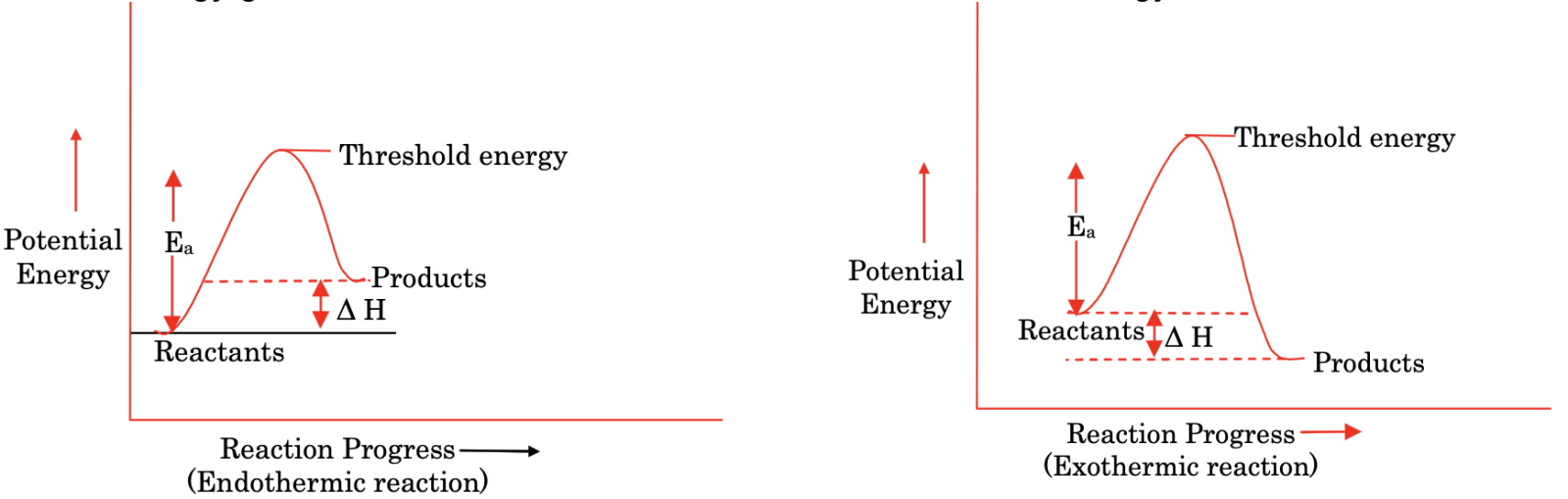
Threshold energy
It is the minimum amount of energy required for a reaction to take place.
Activation energy (Ea)
It is the energy given to the reactant to reach the threshold value of energy.
Arrhenius Equation Notes
Note: Catalyst lowers the activation energy by changing the reaction path and hence increases the rate of reaction.
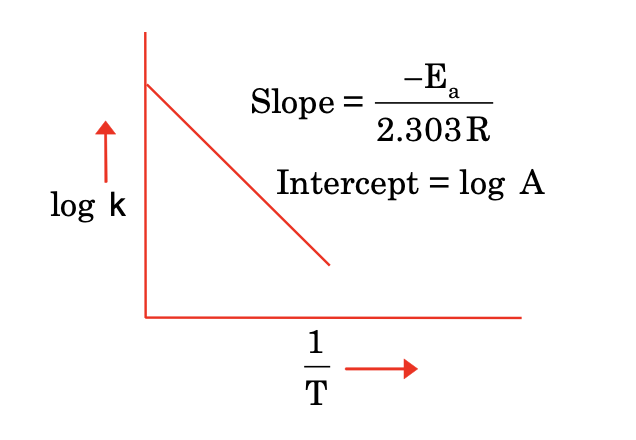
Arrhenius equation
It gives the effect of temperature on rate constant.
where
- Ea – Activation energy (J/mol)
- A – Frequency factor (Arrhenius constant)
- T – Temperature in Kelvin
- R – Gas constant
Taking log, we get
or,
log (k2/k1) = Ea / 2.303 R (1/T1 – 1/T2) where T2 > T1
[Graph: Log k vs 1/T]
Slope = –Ea / 2.303 R
Intercept = log A
PHOTOCHEMICAL REACTIONS
Photochemical reactions takes place in the presence of light, ΔH for such reactions can not be negative.
Temperature has no effect on the rate of such reaction but intensity of light affects the rate.
Photosensitizer
Photosensitizer is the substance which on addition helps to start a photochemical reaction.
Quantum yield of efficiency, Q =
Number of molecules reacting in given time / Total number of quanta absorbed in that time
Example: A chemical reaction was carried out at 300 K and 280 K. The rate constants were found to be k1 and k2 respectively. Then
(A) k2 = 4 k1
(B) k2 = 2 k1
(C) k2 = 0.25 k1
(D) k2 = 0.5 k1
Solution: (C).
For every 10°C rise in temperature doubles the rate constant hence 20°C in temperature decrease will decrease the rate 4 times.
RADIOACTIVITY
It is the phenomena of emission of certain kinds of radiations by some unstable elements.
α – rays
α – rays have charge +2 and mass 4 units, deflected by magnetic and electric field, their penetrating power is low, ionizing power is high, produce fluorescence in ZnS screen.
β – rays
β – rays are same as electrons, their penetrating power is higher than α – rays.
γ – rays
γ – rays have no charge and mass, their velocity is same as electromagnetic radiations, not deflected by electric and magnetic field, their penetrating power is more than β – rays and ionization power is very low.
Note: Nuclear forces are 1021 times stronger than electrostatic forces.
GROUP DISPLACEMENT LAW (GIVEN BY SODDY, FAJAN AND RUSSELL)
(i) Emission of an α–particle results in decrease of two units in atomic number and four units in mass number (new element is two positions on the left in the periodic table).
23892U → 23490Th + 42He (α–particle)
α–decay produces isodiapheres. New element so formed is two positions on the left in the periodic table.
(ii) β–decay results one unit increase in atomic number of daughter element but no change in mass. It produces isobars. New element so formed is one position on the right in the periodic table.
146C → 147N + 0−1e (β–particle)
(iii) γ–rays are emitted after α and β–rays. No change in the position of the element so formed in the periodic table.
Electron does not exist in nucleus, but is emitted by the nucleus by conversion of neutron to proton.
n → p + 0−1e
Radioactive disintegrations follow first order kinetics.
Activity of radioactive element
Activity of radioactive element is its rate of disintegration.
dN/dt = −λ × N
Units of activity are curie (Ci), becquerel (Bq), rutherford (Rd) and disintegrations per second.
1 curie (Ci) = 3.7 × 1010 dps, 1 Bq = 1 dps
1 Rd = 106 dps
Decay constant
For radioactive element decay constant is given by
λ = (2.303 / t) log ( a / (a − x) ) or λ = (2.303 / t) log ( N0 / N )
where N0 is the initial amount and N is the amount left after time t.
t1/2 = 0.693 / λ and taverage = 1 / λ
Rate of disintegration does not depends on temperature.
Packing fraction
Packing fraction = (Isotopic mass − Mass number) / Mass number × 104
It can be −ve, +ve or zero.
Mass defect
Δm = (Σ mproduct − Σ mreactants) amu
Binding energy
Binding energy = Δm × 931.5 MeV, (1 MeV = 1.6 × 10−13 J)
Binding energy per nucleon
Binding energy per nucleon = Binding energy / Mass number
More the binding energy per nucleon, more is the stability.
Any nucleus will be stable when the neutron–proton ratio (n/p) is close to unity.
Important disintegration series
|
Series |
Name of series |
Starting element |
Stable product |
|
4n |
Thorium series |
Th – 232 |
Pb – 208 |
|
4n + 1 |
Neptunium series |
Np – 237 |
Bi – 209 |
|
4n + 2 |
Uranium series |
U – 238 |
Pb – 206 |
|
4n + 3 |
Actinium series |
U – 235 |
Pb – 207 |
When divided by 4 these give remainder of 0, 1, 2 and 3 respectively.
Artificial transmutation
Artificial transmutation is the process of converting one element to another element.
e.g. 147N + 42He → 178O + 11H
The above reaction can be represented as 147N (α, p) 178O.
Best bombarding particles are neutrons because they have no charge.
Artificial or induced radioactivity
Artificial or induced radioactivity is the process of converting stable nuclei to radioactive isotope.
e.g. 42He + 2713Al → 3015P + 10n
(stable) (radioactive)
Cyclotron is used to accelerate bombarding particles (protons, neutrons etc.).
Nuclear fission
It is the process of splitting of a heavier nuclei into smaller atoms by bombarding subatomic particles.
23592U + 10n → 14056Ba + 9336Kr + 3 · 10n
Neutrons emitted by this reaction bombard more uranium atoms and the reaction goes on. It is called chain reaction.
Note:23892U (more abundant isotope) is non radioactive.
Transuranic elements
Transuranic elements come after Uranium and are called synthetic elements.
Nuclear reactor
Nuclear reactor is a device to produce energy by fission reaction of 23595U.
Control rods (made up of boron or cadmium) are used to absorb neutrons and moderators (heavy water or graphite) are used to slow down the speed of neutron.
Breeder reactor
In it the non fissionable material (U238) is first converted into fissionable Pu235 to continue chain reaction.
Atom bomb
Atom bomb is based on the principle of fission reaction.
Nuclear fusion
It is the process in which light elements combine to form bigger atoms. This takes place at very high temperature, i.e. in the sun. Hence, these are called thermonuclear reactions.
4 11H → 42He + 2 0+1e + γ + Energy
Hydrogen bomb
Hydrogen bomb is based on the principle of fusion reaction. Hydrogen bomb involves fission in the centre to start the nuclear fusion reaction.
Note: For the same mass of element energy released in fusion reaction is greater than the energy released in fission reaction.
Applications of radioactivity
- In medicines.
- In carbon dating (to find age of fossils)
146C → 147N + 0-1e
- In geological dating (to find age of minerals).
Tracers
Tracers are the isotopes used in very small quantities to detect the path of reaction.
Neutron activation analysis
In this trace of one element in another is found by activating it with neutrons bombardment.
Magic numbers
The nuclei whose nuclear shells contain 2, 8, 20, 50, 80 or 126 neutrons or protons are more stable. These numbers are called magic numbers.
Spallation reactions
Spallation reactions are similar to fission reactions. They differ in the fact that they are brought about by high energy bombarding particles.