Compounds containing carbonyl group (>C = O) are called carbonyl compounds. These may be of two types:
(a) Aldehydes: The functional group present is R-CHO
(b) Ketones: They can be further classified as simple or mixed ketones depending upon the groups attached.
- Simple ketone:R-CO-R
- Mixed ketone:R-CO-R₁
Where R and R₁ can be an alkyl or aryl group. In carbonyl compounds both carbon and oxygen are sp² hybridized with π electron displaced towards oxygen atom.
Methods of Preparation
From Alkenes
(i) Ozonolysis:
Alkenes are subjected to ozonolysis and subsequent reduction to produce aldehydes and ketones.
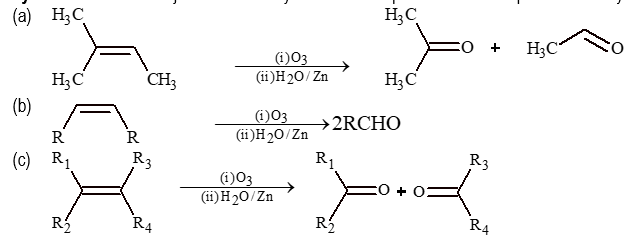
Note: This method is used only for aliphatic carbonyl compounds.
(ii) Oxo process:
This method is used to convert terminal alkenes into carbonyl compounds.
RCH=CH₂ + CO + H₂ → [Co(CO)₄]₂, 150°C, 300 atm → RCH₂CH₂CHO
(iii) Wacker process:
CH₂=CH₂ + PdCl₂/H₂O, air/Cu²⁺Cl₂ → CH₃CHO + Pd + 2HCl
From Alkynes
(i) By hydration of alkynes:
RC≡CH + H₂O, H⁺, HgSO₄ → R-CO-CH₃
From Halides
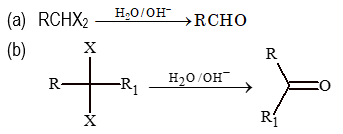
From Alcohols
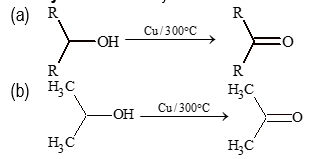
(i) Primary alcohols:
(a) RCH₂OH → Cu,Δ(250°-300°C) or MnO₂/CrO₃/Cr₂O₇²⁻/PCC → RCHO + H₂
b) CH₃CH₂OH → Cu,Δ(250-300°C) → CH₃CHO
Note: Only aldehydes are formed from primary alcohols.
(ii) Secondary alcohols:
Only ketones can be formed from secondary alcohols.
(iii) Oppenauer oxidation:
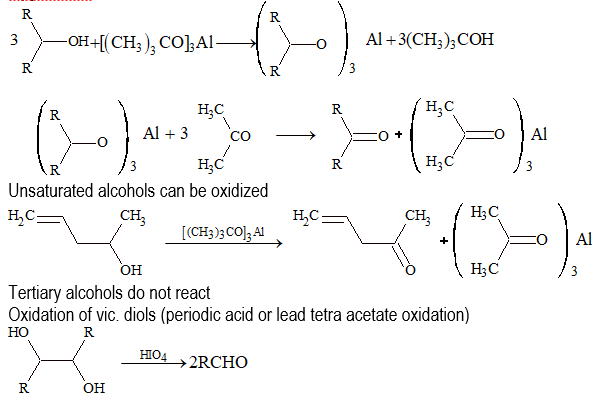
Illustration 1:
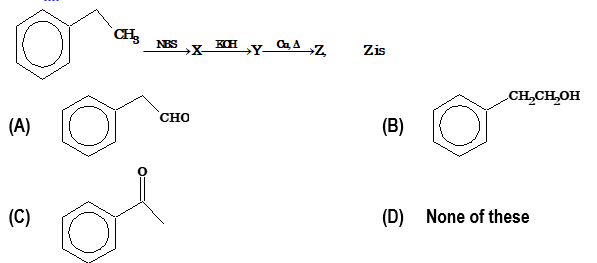
Solution: (C)
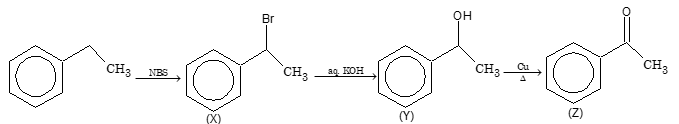
From Carboxylic Acids
(i) Decarboxylation of calcium salts of carboxylic acid:
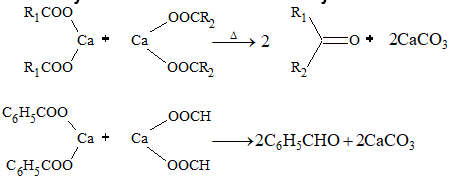
(ii) Decomposition of carboxylic acids:
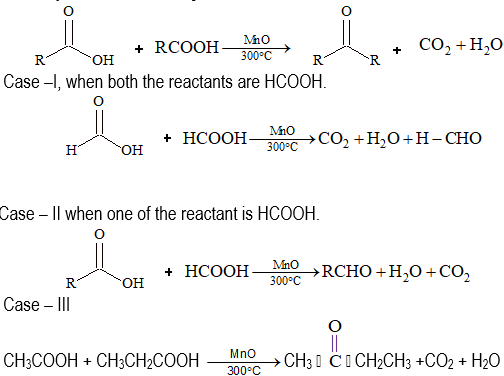
From Acid Chlorides
(i) Preparation of ketones:
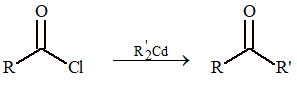
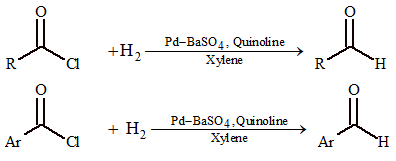
(ii) Rosenmund's reaction:
Acid chlorides on partial reduction in presence of Lindlar's catalyst give aldehydes.
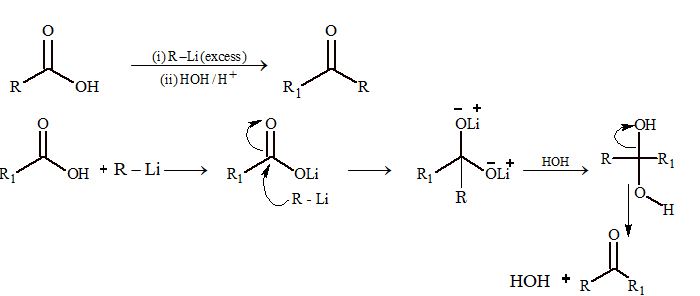
Reaction with Alkyl Lithium
Mechanism:
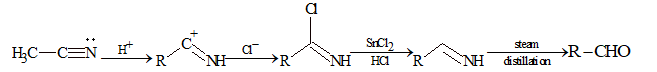
From Cyanides
Stephen reduction:
(i) CH₃C≡N → (i) SnCl₂/HCl → (ii) H₃O⁺ → CH₃CHO
(ii) C₆H₅CN → (i) SnCl₂/HCl → (ii) H₃O⁺ → C₆H₅CHO
Mechanism:
From Grignard Reagent
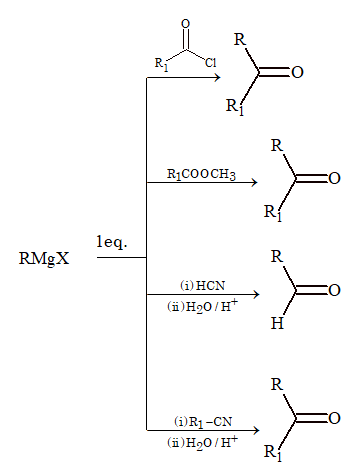
Preparation of Aromatic Carbonyl Compounds
(i) From methyl arenes:
C₆H₅CH₃ → (i) CrO₂Cl₂ → (ii) HOH → C₆H₅CHO
(ii) From chloromethyl arenes:
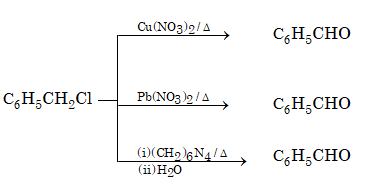
(iii) From benzene:
Gattermann – Koch formylation
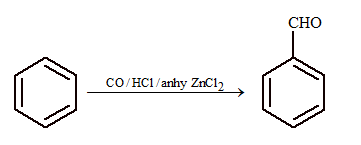
Illustration 2:
Phenyl ethanol can be prepared by reacting benzaldehyde with
(A) methyl bromide
(B) ethyl iodide and magnesium
(C) methyl bromide and aluminium
(D) methyl iodide and magnesium
Solution: (D) CH₃I + Mg → CH₃MgI
C₆H₅CHO + (i) CH₃MgI → (ii) H₂O/H⁺ → C₆H₅CH(OH)CH₃ (1 phenyl ethanol)
Illustration 3:
Reaction between (C₂H₅)₂Cd and CH₃COCl leads to the formation of
(A) dimethyl ketone
(B) ethyl methyl ketone
(C) diethyl ketone
(D) acetaldehyde
Solution: (B)
2CH₃COCl + (C₂H₅)₂Cd → Dry ether → 2CH₃COC₂H₅ + CdCl₂ (ethyl methyl ketone)
Physical Properties
- Aldehydes and ketones have higher boiling points than hydrocarbons of similar molecular weight due to the presence of polar carbonyl group. But aldehydes and ketones have lower boiling point than corresponding alcohols due to lack of intermolecular hydrogen bonding.
- They are soluble in polar solvents like H₂O due to hydrogen bonding.
- Some aromatic aldehydes have pleasant fragrance.
Chemical Properties
The reactions of aldehydes and ketones can be broadly divided into two types depending upon the part of molecule involved in the reaction. The types are,
1. Reactions due to carbonyl group
- Addition reaction
- Oxidation
- Reduction
2. Reactions due to α hydrogen
- (a) Condensation reactions
- (b) Halogenation
Reactions fue to Carbonyl group
Addition Reactions
These are characterized by the nucleophilic attack on the carbon of the carbonyl group.
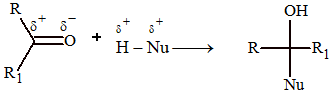
(i) Addition of HCN:
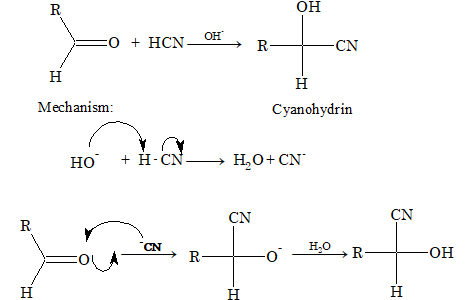
(ii) Addition of NaHSO₃:
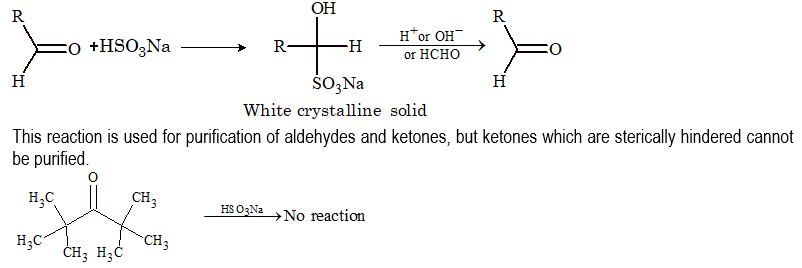
(iii) Addition of alcohols:
Addition of alcohols to carbonyl compounds is catalysed by acid or base.
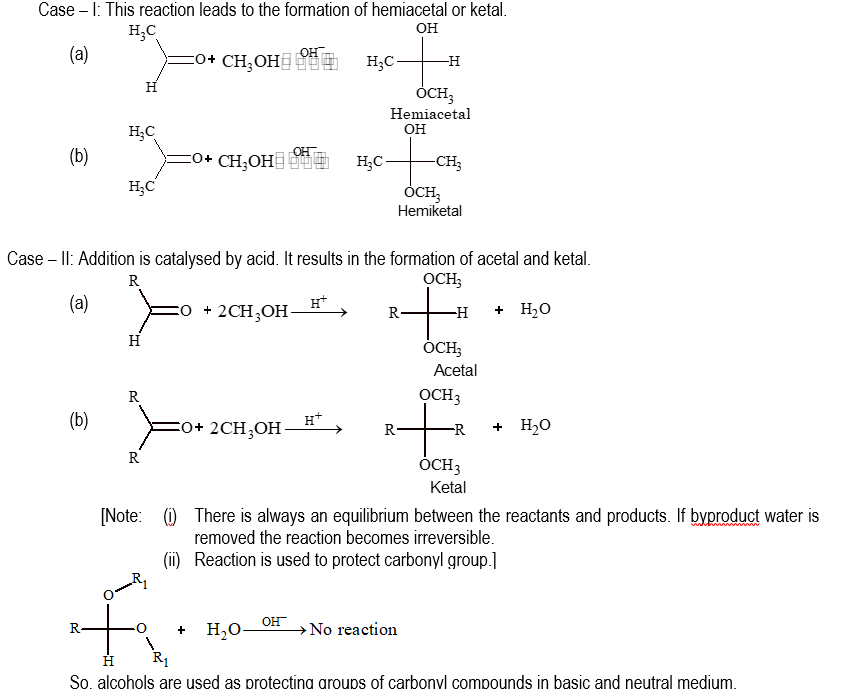
(iv) Addition of Grignard reagent:
Used for the preparation of 1°, 2° and 3° alcohol
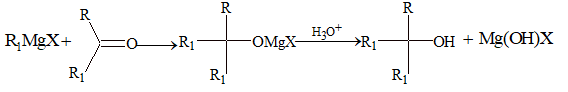
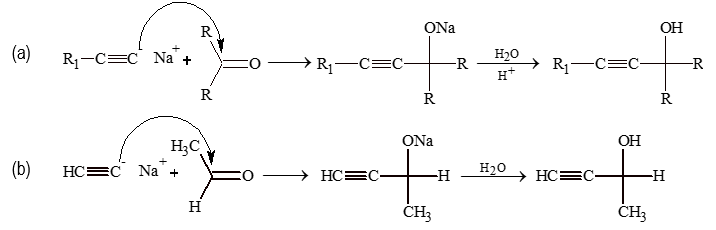
(v) Ethinylation:
The addition of terminal alkynes to the carbonyl compound.
(vi) Addition of ammonia and ammonia derivatives:
In these reactions adducts are formed by primary amines and derivatives of ammonia undergoes elimination to give imine.
R₂CO + H₂NZ → R₂C(OH)NHZ → -H₂O → R₂C=NZ
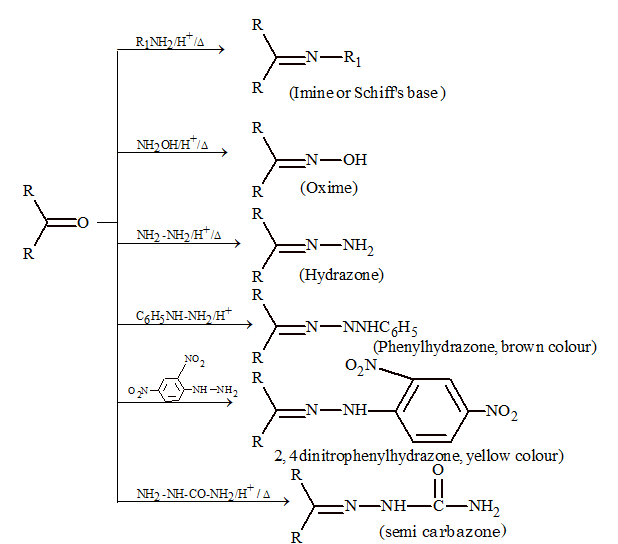
Note: Schiff's base can be used to differentiate between aldehydes and ketones. Schiff's base is pink in colour and is decolourized by the passage of SO₂.
Illustration 4:
CH₃MgBr + H₂C=CH-CHO → H₃O⁺ → X, X is
(A) CH₃CH(OH)CH=CH₂
(B) H₃C-CH₃
(C) CH₃-CH₂-CH₂-CHO
(D) None of the above
Solution: (C)
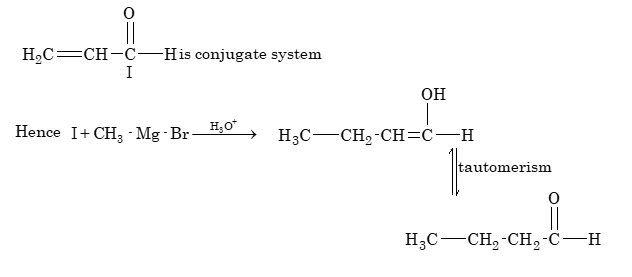
Illustration 5:
Which of the following will react at the slowest rate with a nucleophile?
(A) Methanal
(B) Butanone
(C) 2-pentanone
(D) 3-pentanone
Solution: (D) If the electrophilic character of carbon is considerably reduced, then the reaction will be slow.
Oxidation
1. Oxidation by mild oxidizing agents:
They oxidize aldehydes to carboxylic acids. They can't oxidize ketones.
(i) Fehling's solution:
It is an alkaline solution of cupric ion complexed with sodium potassium tartrate ions.
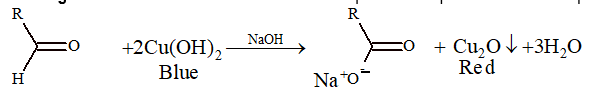
(ii) Benedict's solution:
It is an alkaline solution of cupric ion complexed with sodium potassium citrate ions and the reaction is same as above.
Note: Above two reagents oxidize only aliphatic aldehydes.
(iii) Tollen's reagent:
It is an ammonical silver nitrate solution. It oxidizes only aliphatic aromatic aldehydes.
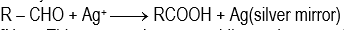
Note: This reagent does not oxidize carbon–carbon multiple bonds.
2. Oxidation by strong oxidising agents:
The below mentioned oxidizing agents can oxidize both aldehydes and ketones to carboxylic acids. The reagents are,
(i) KMnO₄/OH⁻
(ii) KMnO₄/H⁺/Δ
(iii) K₂Cr₂O₇/H⁺/Δ
(iv) conc. HNO₃/Δ
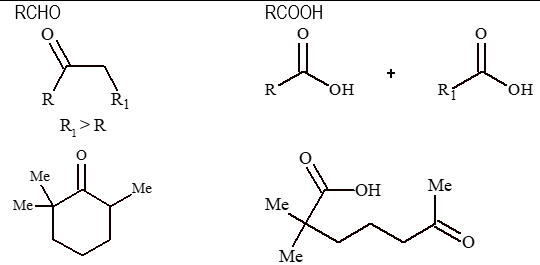
3. Important oxidation reactions:
(i) Haloform reactions:
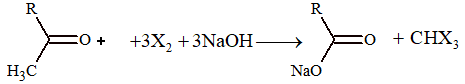
(ii) Oxidation by SeO₂:
SeO₂ oxidized α-CH₂ group into keto group and α-CH₃ group into aldehydic group.
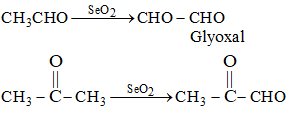
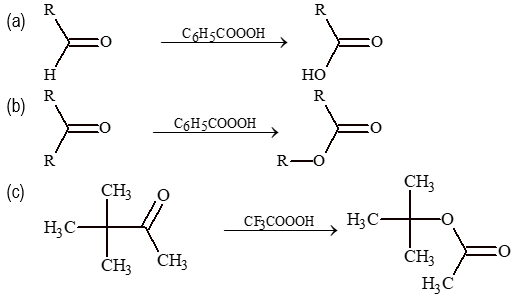
(iii) Baeyer Villiger oxidation:
Reduction
The reduction of carbonyl compounds can be carried out by using various reducing agents. The different reactions are tabulated below:
| Compound | Reagents used | Reduced product |
|---|---|---|
| RCOR₁ (Ketones) | (i) HI/P/Δ (ii) Zn/Hg/conc.HCl (Clemmenson reduction) (iii) NH₂NH₂/OH⁻/Δ (Wolff-Kishner reduction) |
RCH₂–R₁ |
| RCHO (Aldehydes) | (i) LiAlH₄ (ii) NaBH₄ (iii) Aluminium isopropoxide |
R–CH₂–OH |
| RCOR₁ (Ketones) | (i) Mg/Hg/H₂O | RCH(OH)R₁ |
| Aromatic ketones | Na/C₂H₅OH/H₂O | Pinacol (diol) |
Illustration 6:
The end product in the following sequence of reaction is
HC≡CH → HgSO₄/H₂SO₄(dil) → A → CH₃MgBr/H₂O → B → [O] → ?
(A) acetic acid
(B) isopropyl alcohol
(C) acetone
(D) ethanol
Solution: (C)
HC≡CH → Hg²⁺/H₂SO₄(dil) → CH₃CHO → (i) CH₃MgBr/(ii) H₃O⁺ → CH₃CH(OH)CH₃ → [O] → CH₃COCH₃
Illustration 7:
Which of the following will not give iodoform test?
(A) Ethanal
(B) Ethanol
(C) 2-pentanone
(D) 3-pentanone
Solution: (D)
3-pentanone (CH₃CH₂COCH₂CH₃) does not contain CH₃CO group, hence does not give iodoform test.
Reactions due to α-hydrogens
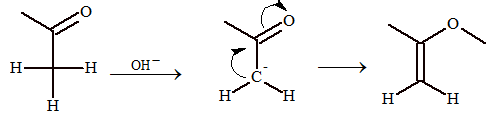
α-hydrogen of carbonyl compounds is acidic in nature.
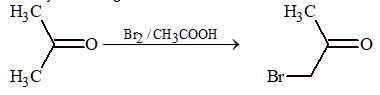
Halogenation:
Carbonyl compounds undergo acid or base catalysed halogenation.
(a) Acid catalysed halogenation
(b) Base catalysed halogenation
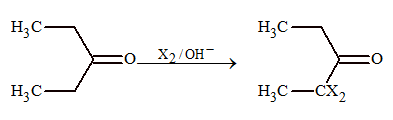
Alkylation by Lithium Enolates
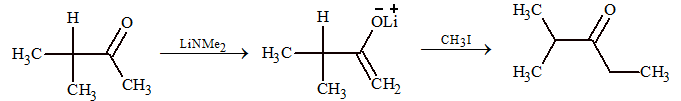
Points to remember:
- α-hydrogen of carbonyl compound is acidic in nature and reacts with base.
- The enolate ion formed is stabilized by resonance.
- Keto form is generally more stable than enol form.
Condensation Reactions
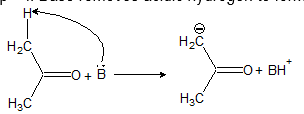
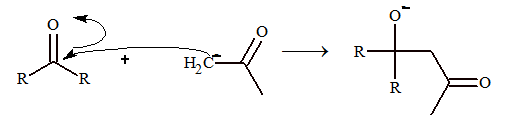
The general steps of condensation reaction are
Step – I: Base removes acidic hydrogen to form carbanion.
Step – II: The carbanion formed acts as a nucleophile and attacks carbon atom of other carbonyl compound.
Step – III: Acceptance of proton.
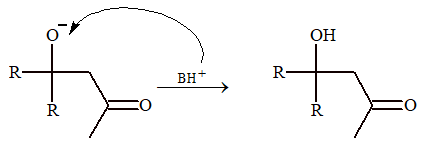
Step – IV: Formation of conjugate bond will bring stabilization, so dehydration occurs.
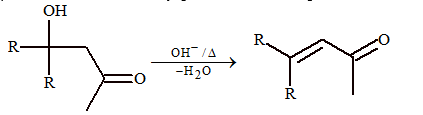
(i) Aldol condensation
Two molecules of carbonyl compounds undergo condensation, where at least one of them possesses acidic hydrogen, i.e. α-H and reaction is carried out in presence of dilute base or dilute acid. The product formed is β-hydroxy carbonyl compound, which is also known as aldol.
CH₃CHO + C₂H₅CHO → OH⁻ → CH₃CH₂CH=CHCHO
Mechanism:
CH₃CHO → OH⁻/-H₂O → CH₂⁻CHO
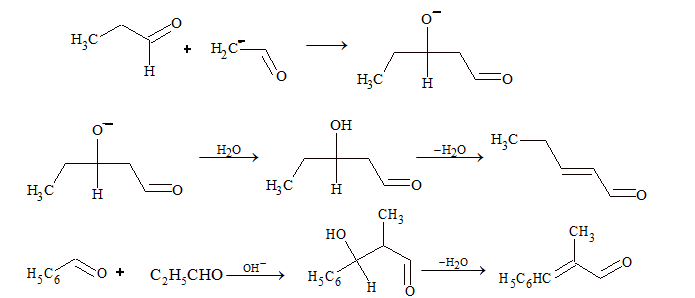
Intramolecular aldol condensation:
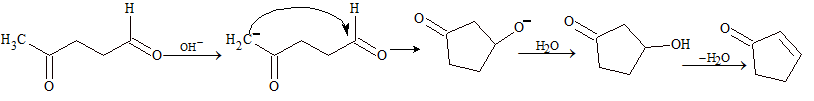
Illustration 8:
Which of the following will be most readily dehydrated in acidic conditions?
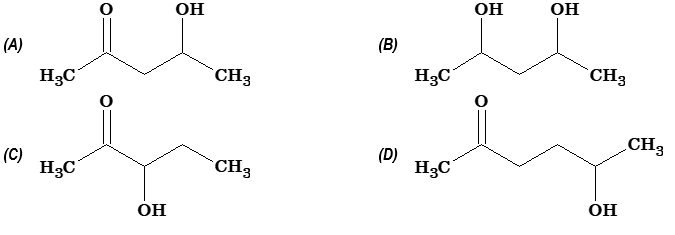
Solution: (A) Aldols, i.e. β-hydroxyaldehydes or β-hydroxyketones readily undergo dehydration to form α,β-unsaturated aldehydes or ketones.
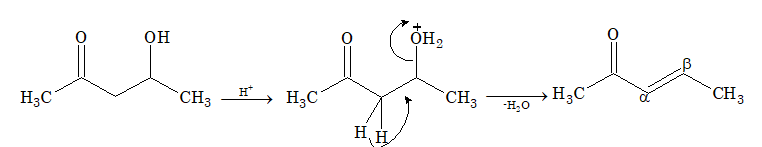
(ii) Cannizzaro reaction:
In the presence of concentrated alkali, aldehydes containing no α-hydrogens undergo self-oxidation and reduction to yield a mixture of an alcohol and a salt of a carboxylic acid. This reaction is known as Cannizzaro reaction.
2C₆H₅CHO → NaOH → C₆H₅CH₂OH + C₆H₅COO⁻Na⁺
Mechanism:
In Cannizzaro reaction two successive additions are involved.
(a) Addition by hydroxide ion in first step
(b) Addition of hydride ion in the next step
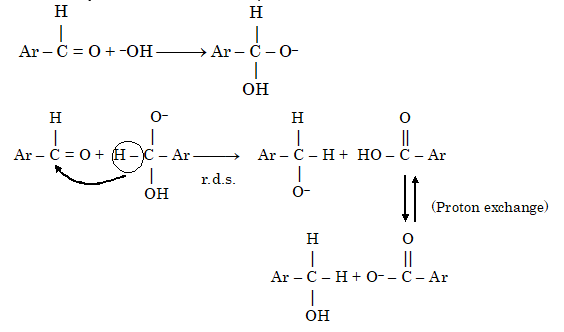
In crossed Cannizzaro reaction, two different aldehydes having no α-hydrogen atoms are involved.
ArCHO + HCHO → HCO₂Na⁺ + ArCH₂OH
(iii) Claisen–Schmidt reaction:
Condensation of an aromatic aldehyde and aliphatic ketone.
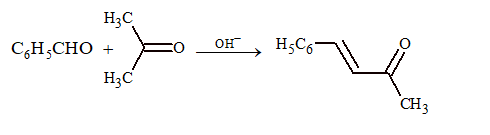
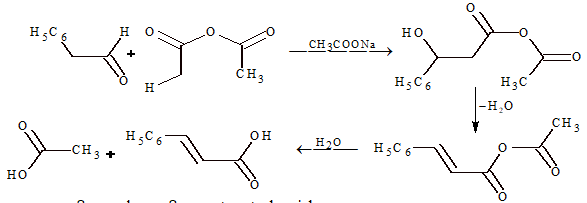
(iv) Perkin's condensation:
This condensation occurs between aromatic aldehyde and aliphatic acid anhydride having at least two α-hydrogen atoms.
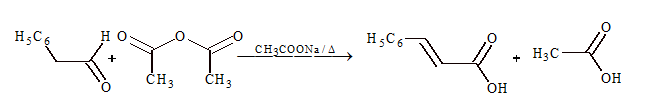
Mechanism:
(v) Knoevenagel Reaction:
This reaction occurs between aromatic aldehyde and compounds having active methylene group. Reaction is catalysed by weak base, e.g. pyridine or piperidine.
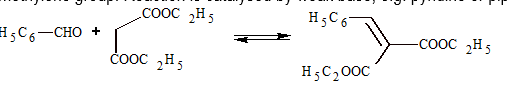
Mechanism:
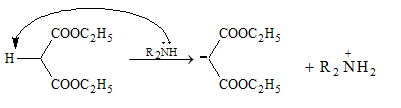
Illustration 9:
A mixture of (CH₃)₃CCHO and HCHO on heating with aqueous NaOH solution will give
(A) HCOONa + (CH₃)₃CCH₂OH
(B) (CH₃)₃CCOONa + CH₃OH
(C) HCOONa + (CH₃)₃CCOONa
(D) CH₃OH + (CH₃)₃C–CH₂OH
Solution: (A) Cross cannizzaro reaction will occur. The carbonyl carbon of formaldehyde being most electrophilic, or most ideal for nucleophilic attack. OH⁻ion (nucleophile) will attack formaldehyde, the product will then donate H⁻to (CH₃)₃CCHO. Thus, formaldehyde will be oxidized to HCOONa and (CH₃)₃CCHO will be reduced to (CH₃)₃CCH₂OH.
Solved Examples
Ques 1. CH₃-CO-CH₃ → SeO₂ → A
A will
(A) reduce Tollen's reagent
(B) gives FeCl₃ test
(C) gives bromine water test
(D) gives benzoin condensation
Sol. (A) Since, A has an aldehyde group, so it reduces Tollen's reagent.
Ques 2. Which of the following gives positive iodoform test and positive Fehling's solution test?
(A) Acetone
(B) Acetaldehyde
(C) Ethanol
(D) Formaldehyde
Sol. (B) Acetaldehyde has CH₃CO– group as well as –CHO group.
Ques 3. The most appropriate reagent for the conversion of 2-pentanone to butanoic acid is
(A) sodium hypoiodite
(B) O₂
(C) acidified KMnO₄
(D) alkaline KMnO₄
Sol. (A) Haloform reaction converts methyl ketones to acids directly.
Ques 4.
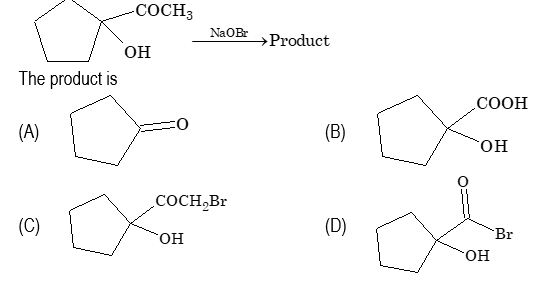
Sol. (B)
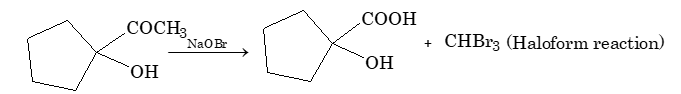
Ques 5.
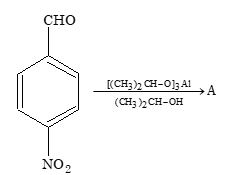
A is
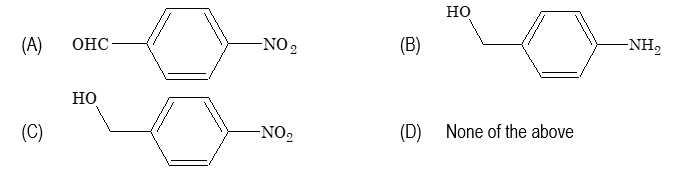
Sol. (C) Aluminium isopropoxide selectively reduces carbonyl group.
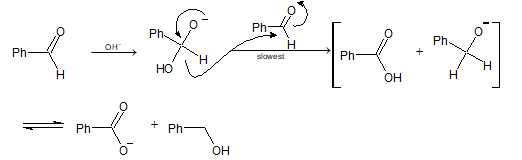
Ques 6. In Cannizzaro reaction given below
PhCHO → OH⁻ → PhCH₂OH + PhCOO⁻
the slowest step is
(A) the attack of OH⁻ at the carbonyl group.
(B) the transfer of hydride to the carbonyl group.
(C) the abstraction of proton from carboxylic acid.
(D) the deprotonation of Ph–COOH.
Sol. (B) Transfer of hydride ion to the carbonyl group is the slowest or the rate determining step.
Ques 7. Which of the following aldehydes is most reactive towards nucleophilic addition reaction?
(A) HCHO
(B) CH₃CHO
(C) C₂H₅CHO
(D) CH₃COCH₃
Sol. (A) HCHO is most reactive towards nucleophilic addition reaction.
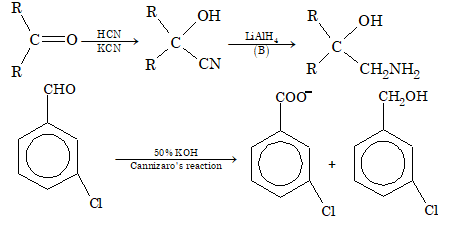
Ques 8. When metachloro benzaldehyde is treated 50% KOH solution, the product(s) obtained is(are)
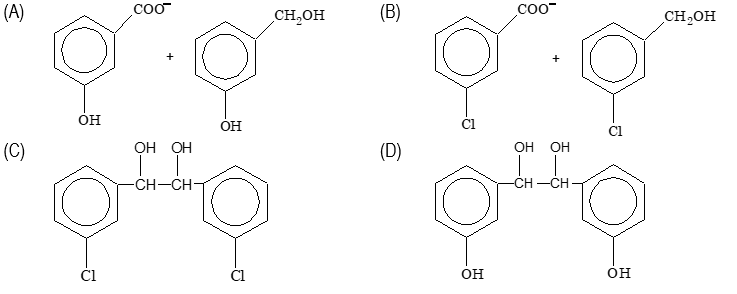
Sol. (B)
Ques 9. Compound 'A' (molecular formula C₃H₈O) is treated with acidified potassium dichromate to form a product 'B' (molecular formula C₃H₆O). 'B' forms a shining silver mirror on warming with ammoniacal silver nitrate. 'B' when treated with an aqueous solution of H₂NCONHNH₂HCl and sodium acetate gives a product 'C'. Identify the structure of 'C'.

Sol. (A)
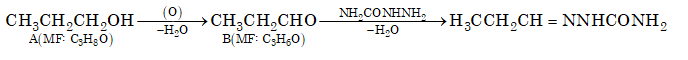
Ques 10. Hydrogenation of benzoyl chloride in the presence of Pd on BaSO₄ gives
(A) benzyl alcohol
(B) benzaldehyde
(C) benzoic acid
(D) phenol
Sol. (B) C₆H₅COCl + H₂ → Pd/BaSO₄ → C₆H₅CHO