The Complete Guide to Heavy Metals Chemistry: From Ores to Industrial Applications
Heavy metals represent some of the most industrially significant elements in the periodic table, playing crucial roles in everything from construction materials to advanced electronics. Understanding the chemistry of these metals—their extraction processes, compound formation, and industrial applications—is fundamental to materials science, metallurgy, and chemical engineering. This comprehensive guide explores the essential chemistry of iron, copper, silver, gold, zinc, mercury, tin, and lead, providing detailed insights into their occurrence, extraction methods, and key chemical properties.
Iron: The Foundation of Modern Industry
Iron stands as the most industrially important heavy metal, with an electronic configuration of [Ar]3d⁶4s² and atomic number 26. This reactive metal occurs primarily in oxide ores including magnetite (Fe₃O₄), hematite (Fe₂O₃), and limonite (Fe₂O₃·3H₂O). The extraction of iron through blast furnace technology represents one of humanity's most significant metallurgical achievements.
The blast furnace process operates through distinct temperature zones, each facilitating specific chemical transformations. In the combustion zone (1500-1600°C), coke burns to produce carbon monoxide, while the reduction zone (250-750°C) converts iron oxides to metallic iron through a series of reduction reactions. The central slag formation zone (800-1100°C) removes silicate impurities, while the fusion zone (1200-1500°C) melts the reduced iron for collection.
Iron's versatility manifests in its three primary commercial forms: cast iron (2.5-4% carbon content), wrought iron (0.12-0.25% carbon), and steel (0.2-1.5% carbon). Steel production utilizes processes like the Bessemer converter and open hearth method, with heat treatment techniques including annealing, quenching, and tempering to develop specific mechanical properties.
Copper: The Conductive Metal
Copper, with electronic configuration [Ar]3d¹⁰4s¹, demonstrates exceptional electrical and thermal conductivity properties that make it indispensable in electrical applications. Primary copper ores include chalcopyrite (CuFeS₂), chalcocite (Cu₂S), and the oxide ore cuprite (Cu₂O). The extraction process involves concentration through froth flotation, followed by roasting, smelting, and Bessemerization.
The roasting process removes volatile impurities while converting sulfides to oxides. Subsequent smelting produces "matte"—a mixture of cuprous sulfide and iron sulfide. Bessemerization then oxidizes remaining iron sulfide and demonstrates auto-reduction, where cuprous oxide reacts with cuprous sulfide to yield metallic copper and sulfur dioxide. The resulting "blister copper" contains approximately 98% copper.
Copper refining employs both oxidative poling and electrolytic methods. Electrolytic refining achieves 99.99% purity using acidified copper sulfate electrolyte, with impure copper anodes and pure copper cathodes. Key copper compounds include cupric sulfate (CuSO₄·5H₂O), used in electroplating and agriculture, and cupric oxide (CuO), utilized in glass manufacturing.
Silver and Gold: The Noble Metals
Silver (electronic configuration [Kr]4d¹⁰5s¹) and gold ([Xe]4f¹⁴5d¹⁰6s¹) represent the noble metals, characterized by their resistance to oxidation and corrosion. Both metals utilize the cyanide process (Mac-Arthur and Forest process) for extraction, forming stable cyano-complexes that facilitate separation from ores.
Silver extraction begins with ore concentration through froth flotation, followed by cyanidation using dilute sodium cyanide solution (0.4-0.6%) in the presence of air. The process forms sodium argento cyanide [NaAg(CN)₂], from which silver precipitates upon zinc addition. Silver refining employs electrolytic methods using silver nitrate electrolyte.
Gold extraction follows similar cyanidation principles, utilizing extremely dilute sodium cyanide solutions (0.03-0.08%). The precious metal's purity is expressed in carats, with pure gold designated as 24 carats. Industrial applications include electronics, jewelry, and specialized chemical processes requiring corrosion-resistant materials.
Zinc, Mercury, Tin, and Lead: Specialized Applications
Zinc (electronic configuration [Ar]3d¹⁰4s²) extraction from zinc blende (ZnS) involves roasting at 1200 K to form zinc oxide, followed by reduction using coke in fire clay retorts. The volatile zinc distills and condenses as crude "zinc spelter." Zinc compounds include zinc oxide (ZnO), known as "philosopher's wool," and zinc sulfate (ZnSO₄·7H₂O), used in lithopone pigment production.
Mercury extraction from cinnabar (HgS) utilizes simple roasting in excess air at 770-780 K, directly producing mercury vapor that condenses to liquid mercury. Mercury compounds include mercurous chloride (Hg₂Cl₂, calomel) and mercuric chloride (HgCl₂, corrosive sublimate), though mercury use has declined significantly due to toxicity concerns.
Tin exhibits enantiotropy among three allotropic forms and finds applications in protective coatings and alloys. Lead forms multiple oxides including litharge (PbO) and red lead (Pb₃O₄), with industrial applications in batteries, radiation shielding, and specialized chemical equipment.
Essential Chemical Formulas for Heavy Metals
| Process/Compound | Formula | Description |
|---|---|---|
| Iron Reduction | Fe₂O₃ + 3CO → 2Fe + 3CO₂ | Primary blast furnace reduction |
| Copper Auto-reduction | Cu₂S + 2Cu₂O → 6Cu + SO₂ | Bessemerization process |
| Silver Cyanidation | Ag₂S + 4NaCN → 2NaAg(CN)₂ + Na₂S | Cyanide process extraction |
| Gold Recovery | 2[NaAu(CN)₂] + Zn → Na₂[Zn(CN)₄] + 2Au | Zinc precipitation |
| Zinc Roasting | 2ZnS + 3O₂ → 2ZnO + 2SO₂ | Ore preparation |
| Mercury Extraction | HgS + O₂ → Hg + SO₂ | Direct thermal decomposition |
| Steel Production | 2FeSO₄ → Fe₂O₃ + SO₂ + SO₃ | Thermal decomposition |
| Litharge Formation | 6Pb + 3O₂ → 6PbO → 2Pb₃O₄ | Lead oxidation |
Industrial Significance and Modern Applications
Heavy metals chemistry continues evolving with advancing technology and environmental considerations. Iron and steel remain fundamental to construction and manufacturing, while copper's exceptional conductivity ensures its continued importance in electrical and electronic applications. Silver and gold maintain their significance in electronics, catalysis, and specialized industrial processes.
Modern metallurgy increasingly emphasizes sustainable extraction methods, recycling technologies, and environmental protection. Understanding the fundamental chemistry of these metals provides the foundation for developing more efficient, environmentally responsible extraction and processing methods that meet growing global demand while minimizing ecological impact.
The study of heavy metals chemistry bridges fundamental chemical principles with practical industrial applications, demonstrating how molecular-level understanding translates to large-scale technological solutions that shape modern society.
Iron (Fe)
Occurrence & Important Ores
Common ores include magnetite (Fe3O4), haematite (Fe2O3), limonite (Fe2O3·3H2O), siderite (FeCO3), and sulphides such as pyrites (FeS2).
Extraction (Blast Furnace Overview)
Iron is extracted mainly from oxide ores via concentration, roasting, and smelting in a blast furnace with coke (reducing agent) and limestone (flux). Distinct temperature zones enable reduction of iron oxides and formation of a protective slag (CaSiO3). The molten product is pig iron, further processed to wrought iron or steel by controlled removal of impurities and carbon adjustment.
Forms of Iron & Heat Treatment
Cast (pig) iron: highest C; wrought iron: lowest C; steel: medium C (including mild, hard, and alloy steels). Heat-treatments such as annealing, quenching, and tempering tune hardness and elasticity.
Key Compounds (Exam Hits)
FeO (basic), Fe2O3 (amphoteric), Fe3O4 (mixed oxide, magnetic); FeSO4·7H2O (reducing agent; forms nitroso complex with NO); FeCl3 (oxidizing, forms Prussian blue with ferricyanide); Mohr’s salt: FeSO4·(NH4)2SO4·6H2O—primary standard for titrations.
Copper (Cu)
Occurrence & Extraction
From sulphide ores (chalcopyrite CuFeS2, chalcocite Cu2S), oxide (cuprite Cu2O), and carbonates (malachite, azurite). Steps: froth flotation → roasting → smelting with silica (slag formation) → Bessemerization (auto-reduction to blister copper) → refining (poling & electrolytic) to 99.99% Cu.
Properties & Compounds
Red-brown, highly conductive; forms green patina (basic copper carbonate) in moist air. Important salts: CuSO4·5H2O (blue vitriol; forms deep blue [Cu(NH3)4]2+), Cu2O (glass red), CuO (black), reactions with KI give Cu2I2 with iodine liberation.
Silver (Ag)
Cyanide Process & Refining
Argentite and related ores dissolve in NaCN (in air) to form NaAg(CN)2; Ag is precipitated with Zn and refined electrolytically (Ag anode → AgNO3 bath). Notable compounds: AgNO3 (lunar caustic; mirror silvering, reagent), AgBr (light-sensitive; photography).
Gold (Au)
Extraction & Purity
Processed similarly by cyanidation with aeration; Au is precipitated with zinc and refined (electrolytic). Purity is expressed in carats (24-carat = pure gold). Gold(III) chloride forms tetrachloroaurate in HCl; Au2S is an insoluble sulphide.
Zinc (Zn)
Ores, Roasting & Reduction
From zinc blende (ZnS), zincite (ZnO), calamine (ZnCO3). Concentrated ore is roasted to ZnO; reduced with coke at high tem
IRON
Symbol: Fe
Atomic number: 26
Electronic configuration: [Ar]3d6, 4s2
It is a reactive metal and does not occur in free state.
Occurrence
1. Oxide ores:
(i) Magnetite (Fe3O4)—It is richest ore of iron.
(ii) Haematite (Fe2O3)
(iii) Limonite (Fe2O3·3H2O)
2. Carbonate ore: Siderite (FeCO3)
3. Sulphide ores:
(i) Iron pyrites (FeS2)
(ii) Chalcopyrites (CuFeS2)
Extraction - The extraction of iron is usually made from its oxide ores, viz., magnetite, haematite and limonite ores involves the following steps:
(i) Concentration of the ore: Crushing and washing with water and then electromagnetic dressing.
(ii) Calcination and Roasting: Heating in excess of air.
- (a) Moisture and carbon dioxide are removed.
Fe2O3·3H2O → Fe2O3 + 3H2O
FeCO3 → FeO + CO2
- (b) Impurities (such as P, S, C, As, Sb etc.) are removed as their volatile oxides.
S + O2 → SO2
- (c) Ferrous oxide is oxidized to ferric oxide.
2FeO + ½ O2 → Fe2O3
Ferric oxide does not form slag at lower temperature so early formation of fusible slag is checked.
- (d) The entire mass becomes porous to facilitate reduction.
(iii) Smelting: The calcined ore mixed with limestone, CaCO3 (a flux) and coke (a reductant) in the ratio 8:1 and charged into the blast furnace from the top. Different temperature zones are formed. The temperature decreases on moving upwards in the blast furnace.
- (a) Combustion zone: It is the lowest zone with a temperature range of 1500–1600°C.
Reactions:
- C + ½ O2 → CO; ΔH = −ve
- CO + O2 → CO2; ΔH = −ve
- CO2 + C —(1500°C)—> 2CO; ΔH = −ve
Reduction zone: It is the upper most zone with a temperature range of 250°C–750°C.
3Fe2O3 + CO 300–400°C → 2Fe3O4 + CO2
Fe3O4 + CO 500–600°C → 3FeO + CO2
3FeO + CO 700°C → Fe (Spongy iron) + CO2
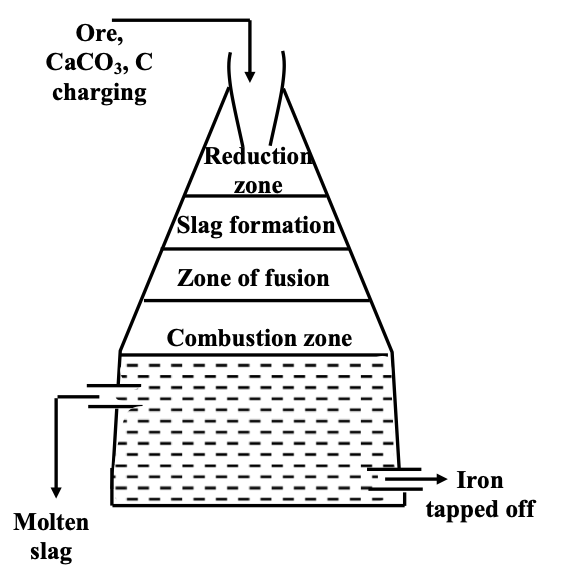
(c) Slag formation zone: It is the central zone with a temperature range of 800–1100°C.
CaCO3——>1000°C CaO + CO2
CaO + SiO2(Sandy impurities)——> CaSiO3(Slag)
(d) Zone of fusion: It is just above the zone of combustion and with a temperature range of 1200–1500°C. The spongy iron from reduction zone melts in this zone and is collected at the bottom, while the slag (CaSiO3) being lighter floats over the molten metal and this prevents the oxidation of Fe by blast of hot air. The molten iron is then allowed to run into boat shaped moulds, called pigs, hence the name pig iron.
Types of Iron
It is available in three commercial varieties.
1. Cast iron or pig iron: Most impure form of iron with highest carbon content (2.5–4%).
2. Wrought iron: Purest form of iron with lowest carbon content (0.12-0.25%).
3. Steel: Most important variety of commercial iron with medium carbon content (0.2-1.5%).
Steel is also available in different varities.
(a) Mild steel: Low carbon content of 0.2-0.5%.
(b) Hard steel: High carbon content of 0.5-1.5%.
(c) Alloy steel: It contains small amount of Ni, Cr, Co, W, Mn, V etc. Stainless steel is an alloy of Fe, Cr and Ni and tool steel is an alloy of Fe, W, V etc.
Steel is generally manufactured from cast iron by
(i) Bessemer process in a large pear-shaped furnace called Bessemer converter.
(ii) L.D process
(iii) Open hearth process, spiegeleisen (alloy of Fe, Mn and C) is added during the manufacture of steel.
Heat treatment of steel:
(i) To develop special properties like hardness, elasticity etc.
(ii) To remove undesirable properties like internal stresses, strains etc.
The various methods used for heat treatment are:
(a) Annealing: Heating steel to redness followed by slow cooling.
(b) Quenching: Heating steel to redness followed by sudden cooling by plunging red hot steel into water or oil.
(c) Tempering: Heating the quenched steel to a temperature much below redness
(473-623 K) followed by slow cooling.
(d) Case hardening: Producing thin coating of hardened steel on the surface of wrought iron or mild steel by heating it in contact with charcoal followed by quenching in oil.
(e) Nitriding: Heating steel at about 700°C in an atmosphere of ammonia resulting in a hard coating of iron nitride on the surface.
Compounds of Iron
1. Oxides
(a) Ferrous oxide, FeO: Obtained when ferrous oxalate is heated to about 430 K in the absence of air.
(COO)2Fe → FeO + CO + CO2
It is a black powder, basic in nature and reacts with dilute acids to give ferrous salts.
FeO + H2SO4 → FeSO4 + H2O
It is used in glass industry to impart green colour to glass.
(b) Ferric oxide, Fe2O3: Obtained by heating carbonate, nitrate or oxalate in the presence of air. It is a reddish brown powder, not affected by air or water and is insoluble in water. It is amphoteric in nature.
Fe2O3 + 6HCl → 2FeCl3 + 3H2O
Fe2O3 + Na2CO3 → 2NaFeO3 + CO2
Fe2O3 + NaOH → 2NaFeO2 + H2O
It is reduced to Fe on heating with C or CO.
Fe2O3 + 3C → 2Fe + 3CO
Fe2O3 + 3CO → 2Fe + 3CO2
It is used as red pigment and also as a polishing powder by jewellers.
(c) Ferrosoferric oxide, Fe3O4 (FeO.Fe2O3): More stable than FeO and Fe2O3, magnetic in nature and dissolves in acid giving a mixture of iron (II) and iron (III) salts.
Fe2O3 + 4H2SO4 → FeSO4 + Fe2(SO4)3 + 4H2O
Ferrous sulphate, FeSO4·7H2O (Green vitriol)
It can be obtained by treating scrap iron with dilute sulphuric acid.
Fe + H2SO4 → FeSO4 + H2
Commercially, it is obtained by exposing big heaps of moist iron pyrites to air when slow oxidation takes place.
2FeS2 + 2H2O + 7O2 → 2FeSO4 + 2H2SO4
- On standing, there is a loss of water of crystallization and also it turns brown on exposure to air due to oxidation to basic ferric sulphate.
4FeSO4 + 2H2O + O2 → 4Fe(OH)SO4
- Strong heating of ferrous sulphate gives ferric oxide with the evolution of SO2 and SO3.
2FeSO4 — Δ → Fe2O3 + SO2 + SO3
- It can reduce MnO4− to Mn2+ and Cr2O72− to Cr3+ in acidic medium. It also reduces nitric acid to nitric oxide, Hg2+ to Hg and Sn4+ to Sn2+.
- It absorbs nitric oxide forming dark brown addition compound, nitroso ferrous sulphate, which is used in the ring test for nitrate ions.
FeSO4 + NO → FeSO4·NO
- It forms double salts of the composition R2SO4·FeSO4·6H2O (where R = an alkali metal or NH4+ radical).
- It reacts with potassium cyanide (excess) forming potassium ferrocyanide.
FeSO4 + 6KCN → K4[Fe(CN)6] + K2SO4
3. Ferric chloride, FeCl₃
Anhydrous ferric chloride is prepared by passing dry chlorine gas over heated iron filings while hydrated ferric chloride (FeCl₃·5H₂O) is prepared by the action of HCl on Fe(OH)₃ or Fe₂(CO₃)₃ or Fe₂O₃.
- Anhydrous FeCl₃ is dark red deliquescent solid and gives acidic solution in water.
FeCl₃ + 3H₂O → Fe(OH)₃ + 3HCl - It is a good oxidizing agent and exists as a dimer in vapour state, Fe₂Cl₆.
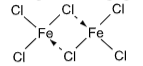
- It forms prussian blue with potassium ferricyanide.
4FeCl₃ + 3K₄[Fe(CN)₆] → Fe₄[Fe(CN)₆]₃ + 12KCl
Ferri–ferrocyanide (Prussian blue)
4. Mohr’s salt, FeSO₄(NH₄)₂SO₄·6H₂O
It is a green coloured double salt used as a primary standard in volumetric analysis.
COPPER
Symbol: Cu
Atomic number: 29
Electronic configuration: [Ar]3d10, 4s1
Occurrence
1. Sulphide ores:
(i) Chalcopyrite (CuFeS2)
(ii) Chalcocite or copper glance (Cu2S)
2. Oxide ore: Cuprite Cu2O - Red colour
3. Carbonate ores:
(i) Malachite CuCO3.Cu(OH)2- Green colour
(ii) Azurite [(2CuCO3)Cu(OH)2] - Blue colour
Extraction
Copper is mostly (about 75%) is extracted from its sulphide ore, copper pyrite, which contains varying amounts of copper and iron sulphides.
Ores which contain copper more than 3% are extracted by dry process or smelting process.
(i) Concentration of ore: By froth floatation process.
(ii) Roasting: Heating strongly in current of air on the hearth of the reverberatory furnace to remove volatile impurities.
Main reaction:
2CuFeS2 + O2 → Cu2S + 2FeS + SO2
Side reaction:
2Cu2S + 3O2 → 2Cu2O + 2SO2 2FeS + 3O2 → 2FeO + 2SO2
(iii) Smelting: The roasted ore is mixed with coke and silica and smelted into a small blast furnace in the presence of excess of air.
(a) The cuprous oxide reacts with ferrous sulphide.
FeS + Cu2O → FeO + Cu2S
(b) Ferrous oxide combines with silica and forms slag.
FeO + SiO2 → FeSiO3 (Slag)
The ‘matte’ mostly cuprous sulphide with a little iron sulphide left at the bottom after the removal of slag is taken out.
Bessemerization:
Molten matte is introduced into a Bessemer converter along with silica and a blast of hot air is blown through the molten mass. The following changes take place:
- (a) Remaining ferrous sulphide gets oxidized.
2FeS + 3O2 → 2FeO + 2SO2 - (b) Ferrous oxide combines with silica to form slag which is drained out.
FeO + SiO2 → FeSiO3 - (c) A part of cuprous sulphide is oxidized which combines with remaining cuprous sulphide to form metal.
2Cu2S + 3O2 → 2Cu2O + 2SO2
Cu2S + 2Cu2O → 6Cu + SO2
This is an example of auto-reduction. The molten copper is poured off and allowed to cool so that the dissolved sulphur-dioxide comes out and large blisters are formed on the surface. Hence, the name ‘blister copper’ with 98% copper and 2% impurities.
Refining:
- (a) By poling: Impure metal is first oxidized whereby impurities are either volatilize or combine with silica forming slag. The small amount of copper is oxidized to cuprous oxide and then reduced by introducing poles of green wood and stirring them vigorously. It gives copper of 99.5% purity.
- (b) By electrolytic refining: The electrolytic bath contains an acidified solution of copper sulphate, impure copper acts as anode while cathode is of pure strip. It gives copper of about 99.99% purity. The impurities settle down below the anode as anode mud.
Properties of Copper
- It has reddish brown colour.
- It is highly malleable and ductile.
- It has high electrical and thermal conductivity.
- In presence of CO2 and moisture, Cu is covered with a green layer of CuCO3.Cu(OH)2.
2Cu + H2O + CO2 + O2 → CuCO3.Cu(OH)2 - It undergoes displacement reactions with less reactive metals, e.g. with Ag. It can displace Ag from AgNO3.
Compounds of Copper
1. Oxides
- (a) Cuprous oxide, Cu2O: It is a reddish brown powder insoluble in water but soluble in ammonia solution, where it forms diamminine Cu (I) ion.
Cu+ + 2NH3 → [Cu(NH3)2]+
It is used to impart red colour to glass. - (b) Cupric oxide, CuO: It is dark black, hygroscopic powder which can be reduced to Cu by hydrogen, CO, etc. It is prepared by heating copper nitrate.
2Cu(NO3)2 —Δ→ 2CuO + 4NO2 + O2
It is used to impart light blue colour to glass.
2. Copper sulphate, CuSO4.5H2O (blue vitriol)
It may be prepared by reacting either CuO or CuCO3 with dilute H2SO4. On an industrial scale, it is obtained by blowing air through a hot mixture of Cu and dilute H2SO4.
2Cu + 2H2SO4 + O2 (air) → CuSO4 + 2H2O
- (i) The salt loses water of crystallization on heating.
CuSO4.5H2O —(100°C)→ CuSO4.H2O —(250°C)→ CuSO4
CuSO4 — strong heat → CuO + SO3
(ii) With ammonia, the cupric hydroxide is first precipitated which dissolves in more of ammonia giving tetraammine copper (II) sulphate complex.
CuSO₄ + 4NH₄OH ⟶ [Cu(NH₃)₄]SO₄ + 4H₂O
(iii) With KI, it gives white ppt. of Cu₂I₂.
4KI + 2CuSO₄ ⟶ 2K₂SO₄ + Cu₂I₂ + I₂
It does not react with KCl, KBr or KF.
(iv) With K₄[Fe(CN)₆], CuSO₄ gives reddish brown precipitate [or chocolate ppt.]
2CuSO₄ + K₄[Fe(CN)₆] ⟶ Cu₂[Fe(CN)₆] + 2K₂SO₄
Reddish brown ppt.
Uses:
- Electroplating and electrorefining of copper.
- In agriculture as a fungicide in Bordeaux mixture [11 parts lime + 16 parts CuSO₄ in 1000 parts of water].
- In dyeing as a mordant.
Cupric sulphide, CuS.
It is prepared as follows:
Cu(NO₃)₂ + H₂S ⟶ CuS + 2HNO₃
Black ppt.
4. Cupric chloride, CuCl₂
It is a dark brown deliquescent solid, readily soluble in water. Its dilute solution is blue in colour due to complex cation, [Cu(H₂O)₄]²⁺, and yellow colour due to complex [CuCl₄]²⁻. The colour of solution however changes to green (due to presence of both complex ions) on addition of conc. HCl.
5. Cuprous chloride, Cu₂Cl₂
It is a white solid, insoluble in water but soluble in excess of hydrochloric acid.
Cu₂Cl₂ + 4HCl → 2H₂CuCl₃
Cu₂Cl₂ + 6HCl → 2H₃CuCl₄
SILVER
Symbol: Ag
Atomic number: 47
Electronic configuration: [Kr]4d10, 5s1
Occurrence
Native state: Associated with copper, gold and platinum metals.
Combined state:
1. Sulphide ores:
(i) Argentite or silver glance (Ag2S)
(ii) Ruby silver (3Ag2S.Sb2S3)
(iii) Silver copper glance [(CuAg)2S]
2. Halide ore: Horn silver (AgCl)
Extraction
The process used for extraction is cyanide process. It is also known as Mac-Arthur and Forest process.
1. Silver compounds dissolve in sodium cyanide solution forming a complex salt, NaAg(CN)2 , in presence of air.
2. Silver is precipitated from this complex salt by the addition of zinc. The process involves the following steps:
(i) Concentration: By froth floatation process.
(ii) Cyanidation: The powdered ore is treated with dilute solution (0.4 to 0.6%) of sodium cyanide in presence of a current of air to form sodium argento cyanide.
Compounds of Silver
1. Silver nitrate, AgNO3 (Lunar caustic)
It is a colourless crystalline compound, soluble in water. It decomposes on exposure to light and thus stored in brown bottles.
2AgNO3 → 2AgNO2 + O2
It can be prepared by heating silver with dilute nitric acid.
3Ag + 4HNO3(dilute, heat) → 3AgNO3 + NO + 2H2O
It is used
(i) for silvering of mirrors.
(ii) as a laboratory reagent.
(iii) in the preparation of inks and hair dyes.
2. Silver bromide, AgBr
It is obtained by adding sodium bromide to silver nitrate solution.
AgNO3 + NaBr → AgBr + NaNO3
The air blown in shifts the equilibrium in forward direction by converting Na2S to Na2SO4.
(iii) Recovery of silver: On putting finely divided zinc, zinc enters the complex ion while Ag precipitates as amorphous mass.
2NaAg(CN)2 + Zn → Na2Zn(CN)4 + 2Ag
(iv) Refining: Silver is refined by electrolysis of silver nitrate solution containing 10% nitric acid using pure silver as cathode and impure silver as anode. Copper, if present as impurity dissolves in the electrolyte and gold, if present is deposited as anode mud.
Compounds of Silver
- Silver nitrate, AgNO3 (Lunar caustic)
It is a colourless crystalline compound, soluble in water. It decomposes on exposure to light and thus stored in brown bottles.
2AgNO3 —Δ→ 2AgNO2 + O2
It can be prepared by heating silver with dilute nitric acid.
3Ag + 4HNO3 (dilute) —heat→ 3AgNO3 + NO + 2H2O
It is used- for silvering of mirrors.
- as a laboratory reagent.
- in the preparation of inks and hair dyes.
- Silver bromide, AgBr
It is obtained by adding sodium bromide to silver nitrate solution.
AgNO3 + NaBr → AgBr + NaNO3
It is a pale yellow solid, insoluble in water and concentrated acids and is partially soluble in strong solution of ammonium hydroxide due to complex formation.
AgBr + 2NH4OH → Ag(NH3)2Br + 2H2O
It is used in photographic solutions as it is most sensitive to light.
2AgBr —light→ 2Ag + Br2
GOLD
Symbol: Au
Atomic number: 79
Electronic configuration: [Xe]4f14, 5d10, 6s1
Occurrence
Some important ores are,
(i) Bismuthaurite (BiAu2)
(ii) Syvanite (AgAuTe2)
(iii) Calverite (AuTe2)
Extraction
The extraction of gold is carried out by using Mac–Arthur and Forest process.
The gold bearing quartz is mined by blasting. The rock is crushed to very fine powder in stamp mills and a pulp of powdered ore and water is made alkaline with slaked lime. The slurry is treated with a dilute solution of sodium cyanide (0.03 to 0.08%) and the solution is agitated by passing air through it.
4Au + 8Na(CN) + 2H2O + O2 → 4[NaAu(CN)2] + 4NaOH
The gold recovered from the solution by precipitation with Zn dust in deaerated cyanide solution.
2[NaAu(CN)2] + Zn → Na2[Zn(CN)4] + 2Au
Gold obtained in this way contains some silver and other impurities.
Refining: The crude gold is made anode and the cathode is pure gold and gold chloride in hydrochloric acid is used as an electrolyte.
Fineness of Gold
Gold is a precious, noble and soft metal. Its bright surface remains untarnished and is therefore used for making ornaments.
To make gold harder, small quantities of other metals, especially copper are added to it. The purity of gold is expressed in carats, pure gold being known as 24 carats.
∴ % of gold in 22 carat gold sample = (22/24) × 100 = 91.66%
Compounds of Gold
- Gold Halide, AuCl₃
It is a reddish solid, soluble in water. It reacts with HCl to give H[Au(Cl)4] which is used in toning process in photography.
HCl + AuCl3 ⟶ H[Au(Cl)4] - Gold sulphide, Au₂S
It is a dark brown solid, insoluble in water. It is prepared as follows:
2K[Au(CN)2] + H2S ⟶ Au2S + 2KCN + 2HCN
ZINC
Symbol: Zn
Atomic number: 30
Electronic configuration: [Ar]3d10, 4s2
Occurrence
Important ores:
(i) Zincite (ZnO)
(ii) Franklinite (ZnO.Fe2O3)
(iii) Zinc blende (ZnS)
(iv) Calamine (ZnCO3)
Extraction:
The extraction of zinc from zinc blende (ZnS) follows the below mentioned steps:
-
Concentration: By froth floatation process.
-
Roasting:
The concentrated ore is roasted at 1200 K in excess of air.
2ZnS + 3O2 → 2ZnO + 2SO2
Some ZnSO4 is also formed but at high temperature the sulphate decomposes to give ZnO.
ZnS + 2O2 → ZnSO4
2ZnSO4 → 2ZnO + 2SO2 + O2
-
Reduction of ZnO:
The oxide is mixed with crushed coke and heated to about 1670 K in fire clay retorts (Belgian process), zinc being volatile distils over and is received in an earthenware pot where it condenses. The crude metal obtained is called zinc spelter.
-
Refining: It is refined by distillation and by electrolytic method.
Compounds of Zinc
-
- Zinc oxide, ZnO (Philosopher’s wool)
Zinc oxide is formed when zinc carbonate, nitrate or hydroxide is strongly heated. It is also obtained by burning zinc in air. It is a white powder and amphoteric in nature.
ZnO + 2HCl → ZnCl2 + H2O
ZnO + 2NaOH → Na2ZnO2 + H2O
- Zinc oxide, ZnO (Philosopher’s wool)
- Zinc sulphide, ZnSO4.7H2O (White vitriol)
It can be prepared by the action of dilute H2SO4 on zinc or its oxide and carbonate. It is used to prepare lithopone, a white pigment. On heating it loses its molecules of water as:
ZnSO4.7H2O 375K → ZnSO4.H2O 725K → ZnSO41075K → ZnO + SO2 + O2
MERCURY
Symbol: Hg
Atomic number: 80
Electronic configuration: [Xe]4f14, 5d10, 6s2
Occurrence
Important ore: Cinnabar (HgS)
Extraction
(i) Concentration of the ore: By froth floatation process.
(ii) Roasting: The concentrated ore is roasted in the presence of excess of air at 770 to 780 K to form HgO, which further decomposes to mercury vapours and oxygen.
2HgS + 3SO2 → 2HgO + 2SO2
2HgO → Hg + O2
(iii) Refining: It is refined by filtering impure mercury through thick canvass. It is then dropped into 5% nitric acid solution where metallic impurities like Fe, Cu, Zn etc., get converted into their respective nitrates and go into solution and mercury free from impurities is received in the receiver. It is further refined by distillation under reduced pressure. More volatile mercury is distilled first.
Compounds of Mercury
1. Mercurous chloride, Hg2Cl2 (Calomel)
It is a white solid insoluble in water. It turns black when treated with ammonium hydroxide forming a mixture of mercury and mercuric amino chloride.
Hg2Cl2 + 2NH4OH → Hg(NH2)Cl + Hg + NH4Cl + 2H2O
Black
It is used as a purgative in medicine.
2. Mercuric chloride, HgCl₂ (Corrosive sublimate)
It is a colourless solid, sparingly soluble in water. Aqueous ammonia gives a white precipitate of mercuric amino chloride with HgCl₂ solution.
HgCl₂ + NH₄OH → Hg(NH₂)Cl + NH₄Cl + 2H₂O
3. Mercuric iodide, HgI₂
- It is a yellow solid below 400 K but changes to red solid above 400 K.
HgI₂ (Red) ⇄ HgI₂ (Yellow) (at 400 K) - It dissolves in excess of KI forming K₂HgI₄.
HgI₂ + 2KI → K₂HgI₄
Alkaline solution of K₂HgI₄ is called Nessler’s reagent.
TIN
Symbol: Sn
Atomic number: 50
Electronic configuration: [Kr4d10, 5s2, 5p2]
Occurrence
Tin exhibits enantiotropy among its three allotropic forms.
Grey tin → White tin → Rhombic tin
It liberates H2 with dilute HCl and H2SO4; with hot concentrated H2SO4 it liberates SO2.
Sn + 2SO42− + H+ ⟶ Sn4+ + 2SO2 + 4H2O
Tin also dissolves in hot alkaline forming stannates and liberating hydrogen.
Compounds of Tin
1. Stannous oxide, SnO
It is a dark grey or black powder. It is an amphoteric oxide and dissolves in both acids and alkalis.
SnO + 2HCl ⟶ SnCl2 + H2O
SnO + 2NaOH ⟶ Na2SnO2 + H2O
Stannite ion acts as a powerful reducing agent. For example, MnO4− is reduced to MnO2 and Bi3+ ions to Bi.
2. Stannic oxide, SnO2
It is a white powder and does not react with common acids except sulphuric acid. Tin dioxide is also soluble in alkalis.
SnO2 + 2KOH ⟶ K2SnO3 + H2O
3. Stannous chloride, SnCl2.2H2O
It is soluble in water. However, on standing, the precipitate of Sn(OH)Cl is obtained. Stannous chloride in concentrated HCl is a powerful reducing agent. With ammonia, SnCl2 forms a number of double salts (SnCl2.NH3, SnCl2.2NH3 and 3SnCl2.2NH3)
4. Stannic chloride, SnCl4
It is a colourless fuming liquid. With limited water, it form a series of hydrated salts (SnCl4.3H2O, SnCl4.5H2O, SnCl4.6H2O and SnCl4.8H2O). With excess water it is hydrolysed.
LEAD
Symbol: Pb
Atomic number: 82
Electronic configuration: [Xe]4f14, 5d10, 6s2, 6p2
Occurrence
It is a soft, bluish-grey and highly malleable metal. Dry air has no action on lead but in moist air a protective coating of basic carbonate is formed and protects it from further oxidation. When heated in air, it forms litharge which at very high temperature is converted to red lead.
6Pb + 3O2 →(Litharge, T>725K, O2) 6PbO →(Red lead) 2Pb3O4
Compounds of Lead
1. Oxides:
Lead forms five oxides, namely, monoxide (PbO), dioxide (PbO2), mixed oxide (Pb3O4, red lead), suboxide (Pb2O), and sesquioxide (Pb2O3).
(i) Lead monoxide is amphoteric in nature.
PbO + 2HNO3 → Pb(NO3)2 + H2O
PbO + 2NaOH → Na2PbO2 + H2O
(ii) Lead dioxide is a chocolate brown powder and is a powerful oxidizing agent. It dissolves in concentrated HCl as well as in concentrated NaOH.
(iii) Red lead is used as a protective paint for iron and steel and as an oxidizing agent in laboratory.
(iv) Lead suboxide is obtained when lead oxalate is heated in absence of air.
2PbC2O4 → Pb2O + 3CO2 + CO
(v) Lead sesquioxide (Pb2O3) is obtained when lead monoxide is heated in air up to 775 K.
4PbO + O2 → 2Pb2O3
Solved Example
1. The substance that sublimes on heating is
(A) MgCl2
(B) AgCl
(C) HgCl2
(D) NaCl
Sol. (C).
2. The purple colour of [Ti(H2O)6]3+ ion is due to
(A) unpaired d-electron
(B) transfer of an electron
(C) intermolecular vibrations
(D) presence of water molecules
Sol. (A).
3. When calomel reacts with ammonium hydroxide we get
(A) HgNH2Cl
(B) NH2–Hg–Hg–Cl
(C) Hg2O
(D) HgO
Sol. (A).
4. Transition elements are coloured
(A) due to small size
(B) due to metallic nature
(C) due to unpaired d-electrons
(D) all of the above
Sol. (C).
5. A white powder soluble in NH4OH but insoluble in water is
(A) BaSO4
(B) CuSO4
(C) PbSO4
(D) AgCl
Sol. (D).
6. Which of the following transition metal cation has maximum unpaired electrons?
(A) Mn2+
(B) Fe2+
(C) Ni3+
(D) Cu+
Sol. (A).
7. Which of the following ore is not an ore of iron?
(A) Haematite
(B) Magnetite
(C) Cassiterite
(D) Limonite
Sol. (C).
8. The colour of FeSO4(NH4)2SO4.6H2O is
(A) red
(B) white
(C) blue
(D) green
Sol. (D).
9. Iron is manufactured from the ore
(A) cryolite
(B) bauxite
(C) haematite
(D) chalcopyrite
Sol. (C)