Ace your CBSE Science and Chemistry exams with in-depth, easy-to-understand lessons on Ores and Metallurgy. Get expert guidance, practical examples, and exam-oriented notes designed to make learning effortless and scoring high inevitable.
Why Choose Our Ores & Metallurgy Course?
Whether you’re struggling with definitions like ores, minerals, and gangue, or need clarity on extraction methods like froth flotation, magnetic separation, calcination, roasting, and smelting, we break down every concept into simple steps. Our curriculum aligns perfectly with CBSE guidelines, ensuring you study exactly what matters for your exams.
Ores & Mettallurgy Comprehensive Curriculum
-
Types of Ores – Native, Silicates, Oxides, Carbonates, Sulphides, Halides, Sulphates, Phosphates.
-
Metallurgical Processes – Concentration, Extraction, Refining.
-
Real-life Examples & Tables – Chief ores of Aluminium, Copper, Zinc, Lead, Iron, and more.
-
Illustrations & Solved Examples – Understand through step-by-step worked problems.
-
Exam-Oriented Exercises – Practice questions based on actual CBSE patterns.
How We Teach
Using flowcharts, diagrams, and CBSE-specific tips, we make topics like electrolytic refining, froth flotation process, and aluminothermic reduction easy to learn and revise. Our mind maps and quick notes make last-minute preparation stress-free.
INTRODUCTION
Metals have an extensive usage in daily life. The main source of metals is the earth crust. The extraction of metal is complicated process which involves step-by-step different methods. The common terms used in the process of extraction are follows:
Minerals. The naturally occurring chemical substances in the earth crust which are obtained by mining are called minerals.
Ores. The minerals which can be used as a source for commercial recovery of a desired metal are called ores.
Gangue. The undesired earthly materials associated with ores are called gangue.
All the ores are minerals but all minerals are not ores.
Some important types of ores are listed in Table 1.
Table.1 Important types of ores
Ore Types and Examples
| Ore Type | Examples |
|---|---|
| Native | Cu, Ag, Au, Hg, As, Bi, Sb, Pd, Pt, S, noble gases |
| Silicates | Be3Al2Si6O18 (beryl), Zn2SiO4, Sc2Si2O7 (thortveitite), NiSiO3, MgSiO3 |
| Oxides | Al2O3·H2O (diaspore), Al2O3·H2O (bauxite), Fe2O3 (haematite), Fe3O4 (magnetite), SnO2 (cassiterite or tinstone), MnO2 (pyrolusite), TiO2, FeCr2O4 (iron chromite), WO3, Cu2O, ZnO (zincite) |
| Carbonates | CaCO3 (calcite), CaCO3·MgCO3 (dolomite), FeCO3 (siderite), PbCO3, BaCO3, SrCO3, ZnCO3 (calamine), MnCO3, CuCO3·Cu(OH)2 (malachite) |
| Sulphides | Ag2S (silver glance or argentite), Cu2S (copper glance or chalcocite), copper pyrites (CuFeS2), PbS (lead glance), ZnS (zinc blende), FeS, Bi2S3, NiS, CaS, MoS3 |
| Halides | NaCl, KCl, AgCl (silver glance), MgCl2 (in sea water) |
| Sulphates | BaSO4, SrSO4, PbSO4, CaSO4·2H2O (gypsum) |
| Phosphates | CePO4, LaPO4, Th3(PO4)4, LiF·AlPO4 |
Some important chief ores of some metals are listed in the Table 2.
Table 2. Chief ores of some metals
| Metals | Name of chief ores |
|---|---|
| Aluminium | Bauxite, Al2O3·xH2O; Diaspore, Al2O3, H2O |
| Chromium | Chromite, FeIICr2IIIO4 (as chrome iron stone) |
| Copper | Copper pyrites, CuFeS2; Malachite, CuCO3·Cu(OH)2 |
| Iron | Haematite, Fe2O3; Magnetite, Fe3O4 |
| Manganese | Pyrolusite, MnO2 |
| Tin | Cassiterite, SnO2 (as tin stone) |
| Zinc | Zinc blende, ZnS; Calamine, ZnCO3 |
| Lead | Galena, PbS |
EXTRACTION OF METALS
The process of extraction of a metal in pure form from its ore is known as metallurgy.
There are three main stages of metallurgy. They are
(i) concentration of ore.
(ii) extraction of crude metal from the concentrated ore.
(iii) refining of crude metal.
Different methods are available for each of these stages. The choice of the method employed, in a particular case, depends on factors such as the type of impurity, the type of metal, available conditions, etc.
Concentration of Ores
Removal of earth matter, rock matter, sand, lime stone etc. from ores is called dressing or concentration. Following methods are commonly used for concentration of ore.
Levigation or gravity separation or hydraulic washing method. This method is based on the difference in specific gravity of the gangue particles and ore particles.
This method is used when the ore particles are heavier than the earthy or rocky gangue particles. The oxide ores such as those of iron (haematite), tin (tin stone) and native ores of Au, Ag etc. are usually concentrated by this method. The process is carried out in specially designated tables called Wilfley tables.
Magnetic concentration method. The method is applicable when either ore or gangue has strong ferromagnetic nature, e.g. iron, tin.
For example, chromite, (FeO.Cr2O3 = FeCr2O4) - an ore of chromium, magnetite (Fe3O4) - an ore of iron and pyrolusite (MnO2) - an ore of manganese being magnetic are separated from non-magnetic silicious gangue by this method. Similarly, tinstone or cassiterite (SnO2), an ore of tin being non-magnetic can be separated from magnetic impurities like those of tungstates of iron and manganese which are generally associated with it, by this method.
Froth floatation process.
This method is widely used for the concentration of sulphide ores such as zinc blende (ZnS), copper pyrites (CuFeS2), galena (PbS) etc. This method is based upon the fact that the surface of sulphide ores is preferentially wetted by oils while that of gangue is preferentially wetted by water.
(i) The process is based on the difference in wetting nature of ore and gangue particles.
(ii) Sulphides ores are mainly dressed by this process.
(iii) Finely powdered ore is mixed with water and small quantity of pine oil. The mixture is agitated by passing compressed air through it. The oil reduces the surface tension and the concentrated ore preferentially wetted by oil comes on top with the froth leaving behind heavy gangue matter wetted by water.
Extraction of Crude Metal from Concentrated Ore
Calcination. It involves simple decomposition of ore on heating below its melting point usually in absence of air to produce new compounds having higher percentage of metal as well as removing the moisture, organic matter and volatile impurities, e.g. CO2, SO2 etc. Calcination makes the ore porous and is made in reverberatory furnace.
Gangue
CuCO3·Cu(OH)2 —Δ→ CuO + CO2 + H2O
Malachite
Fe2O3·3H2O —Δ→ Fe2O3 + 3H2O
Haematite
Roasting. It involves the action of heat in limited supply of air on ore below its melting point to produce other chemical changes along with decomposition.
(i) The ore looses S, Se, As, Sb as oxides leaving behind oxides of metals.
- 2CuFeS2 + O2 → Cu2S + 2FeS + SO2↑
- 2FeS + 3O2 → 2FeO + 2SO2
- 2Cu2S + 3O2 → 2Cu2O + 2SO2
Leaching. It involves the treatment of the ore with suitable reagent as to make it soluble while impurities remains insoluble. Alumina (Al2O3) dissolves in NaOH forming soluble sodium meta aluminate while ferric oxide, titanium oxide and silica remain as insoluble part.
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Smelting. The phenomenon in which ore is mixed with suitable flux and coke and is heated to fusion is known as smelting.
Cupellation. The process in which impure sample of metal (say Pb in Ag) is fused in a bone ash crucibles (cupel) on the hearth of furnace in a blast of air. The impurity (Pb) present is oxidized and blown away with air. Some PbO is absorbed by the cupel.
Example: The reaction 2ZnS + 3O2-> 2ZnO + 2SO2 in the metallurgical process of zinc is called
(A) calcination
(B) cupellation
(C) smelting
(D) roasting
Solution: (D)
Example: Composition of carnallite is
(A) Na3AlF6
(B) KNO3.MgNO3.6H2O
(C) KCl.MgCl2.6H2O
(D) none of the above
Solution: (C)
Example: Which of the following is an alloy of aluminium?
(A) Al clad
(B) Duralumin
(C) Magnalium
(D) All
Solution: (D).
Example: Which of the following is malachite ore?
(A) Cu2S
(B) Cu2O
(C) CuCO3.Cu(OH)2
(D) CuCO3
Solution: (C).
Extraction of Free Metals
- Carbon reduction process: the oxides of less electropositive metals like Pb, Fe, Zn, Sb and Cu are reduced by strongly heating with coal or coke.
Fe2O3 + 3C → 2Fe + 3CO
ZnO + C → Zn + CO
- Air reduction or self reduction: Metal oxides with less active metals, e.g. Hg, Cu and Pb are easily reduced with air. The ores are roasted in air to separate metal. The process is named as auto reduction.
-
2HgS + 3O2 → 2HgO + 2SO2(Cinnabar)
2HgO + HgS → 3Hg + SO2
-
2Cu2S + 3O2 → 2Cu2O + 2SO2(Copper glance)
2Cu2O + Cu2S → 6Cu + SO2
-
2PbS + 3O2 → 2PbO + 2SO2(Galena)
2PbO + PbS → 3Pb + SO2
-
(iii) Reduction with powerful reducing agents:
Extraction of less electropositive metals say Cr, Mn, Cu, Ni etc. can be made by heating their oxides with strong reducing agents, e.g. CO, CO + H2, Na, Al, Mg. If Al is used as reducing agent, the process is known as Gold Schmidt–Aluminothermic process and the mixture containing ore and Al is known as themite process.
Cr2O3 + 2Al ——→ Al2O3 + 2Cr
(Thermite)
3Mn3O4 + 8Al ——→ 4Al2O3 + 9Mn
CuO + CO ——→ Cu + CO2
CaI2 + 2K ——→ Ca + 2KI
2NiO + CO + H2 ——→ 2Ni + CO2 + H2O
The Gold Schmidt–Aluminothermic process is commonly used for those metals which have high melting point and are to be extracted from their oxides and their reduction with carbon is not satisfactory.
(iv) Electrolytic reduction:
Highly electropositive elements are obtained by the electrolysis of their oxides, hydroxides or chlorides in fused state.
NaCl (fused) ——→ Na+ + Cl−
At anode: Cl− ——→ ½ Cl2 + e−
At cathode: Na+ + e− ——→ Na
A small amount of some other salt say MgCl2 are added to ore, in order to lower its fusion point and enhance conductance.
(v) Amalgamation process: Noble metal ores like Ag, Au, Pt in finely powdered state are mixed with water to form slurry. Then it is mixed with Hg to form amalgam. The metal is recovered from amalgam by distillation.
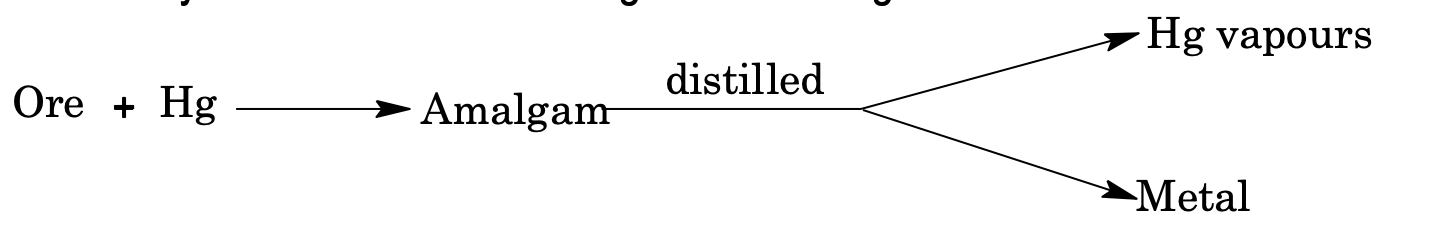
(vi) Hydro metallurgical process
This method is based on the fact that more electropositive metals can displace less electropositive metals from its salt solution. This method is also known as complex salt formation method.
Ag2S + 4NaCN → 2Na[Ag(CN)2] + Na2S
2Na[Ag(CN)2] + Zn → 2Ag + Na2[Zn(CN)4]
Slag and flux. Non-fusible impurities are converted into fusible (slag) mass by adding a suitable substance known as flux. The slag floats over the molten metal and is skimmed off from the surface.
Mineral + Non-fusible mass + Reducing agent + Flux —Δ→ Metal + Slag + Gases
The nature of flux used depends upon the nature of impurities to be removed. An acidic flux (silica, borax) is used to remove basic impurities like FeO, CaO etc.
Reaction (acidic flux removing basic impurity):
SiO₂ + CaO → CaSiO₃
(“SiO₂” = acidic flux, “CaO” = basic impurity, product CaSiO₃ = slag)
A basic flux (CaCO₃, MgCO₃, Fe₂O₃ etc.) is used to remove the acidic impurities like SiO₂.
Reaction (basic flux removing acidic impurity):
CaCO₃ + SiO₂→ CaSiO₃ + CO₂
(“CaCO₃” = basic flux, “SiO₂” = acidic impurity, product CaSiO₃ = slag)
Example: The matte is impure substance of
(A) Cu
(B) Fe
(C) Pb
(D) Al
Solution: (A)
Example: Extraction of Ag from commercial lead is possible by
(A) Parke’s process
(B) Clarke’s process
(C) Pattinson’s process
(D) Electrolyte process
Solution:(A)
Example: In the metallurgy of iron, when limestone is added to the blast furnace, the calcium ion ends up as
(A) slag
(B) gangue
(C) metallic calcium
(D) calcium carbonate
Solution: (A).
Refining of Crude Metal
The metals obtained by either of the above operations is known as crude form. The crude form is usually contaminated with impurities of
(i) other metals obtained by simultaneous reduction of their respected oxides present in the ore as impurities.
(ii) non-metals like silicon or phosphorous formed by reduction in the furnace.
(iii) unreduced oxides and sulphides of the metals.
(iv) residual slag, flux etc. introduced during treatment in furnace.
Liquation process. The refining process for crude metal based on the difference in fusibility of metal and impurities is named as liquation process. Zn-Pb mixture is separated by heating the crude form just above the melting point of Zn where Pb remains as non-fusible mass. The molten mass is allowed to flow on an inclined plane where non fusible mass is left behind.
Distillation method. Volatile metals (Hg, Zn, Cd) are easily purified by distillation. The impure metal is heated in a retort and vapours of volatile metals are collected and condensed in a receiver leaving behind non-volatile impurities.
Oxidation process. Impurities in crude form having more affinity for O2 than the metal itself are oxidized in suitable furnace. The oxides formed at the surface are skimmed off. The various oxidation process used for different metals are poling, pudding, bessemerization and cupellation.
Electrolytic refining. In an electrolytic cell the impure metal is made anode and the pure metal plate is made cathode. The solution consists of same metal salt. On electrolysis, pure metal from the crude form dissolves at anode whereas at cathode pure metal is deposited. The soluble impurities pass into the solution while the insoluble ones are collected below the anode as anode mud or anode sludge.
Heating of crude form with ore. Fe and Sb ores are heated with crude form which removes the contaminated reducing agents (S and C) with it and pure metal is obtained.
Zone refining. The principle of zone refining is based on the fact that the impurities are more soluble in molten state than pure metal. A circular mobile heater is fixed at one end of a rod of the impure metal and the heater is allowed to move slowly above the metal. The melted zone moves along with the heater. As the heater moves forward, the pure metal crystallizes while the impurities move forward with the molten zone.
Highly pure elements like Ge, Si, B, Ga, In etc. are purified by this method. It is a very useful technique for producing semiconductors.
Vapour phase refining. This process is used in the refining of Ni. Nickel on heating in a stream of CO forms volatile nickel carbonyl [Ni(CO)4]. Its vapour on further heating to higher temperature undergoes thermal decomposition to produce pure nickel.
Ni + 4 CO → Ni(CO)4(350 K)
Ni(CO)4 → Ni + 4 CO (450 K)
This process is called Mond’s process. A similar process called the Van Arkel process is used to purify zirconium and titanium.
On heating Zr in iodine vapour, volatile zirconium iodide, ZrI4, is formed. On further heating over a tungsten filament, ZrI4 undergoes thermal decomposition to produce pure Zr.
Zr + 2 I2 → ZrI4(870 K)
ZrI4 → Zr + 2 I2(2025 K)
Flow Sheet Diagram for General Metallurgical Operations
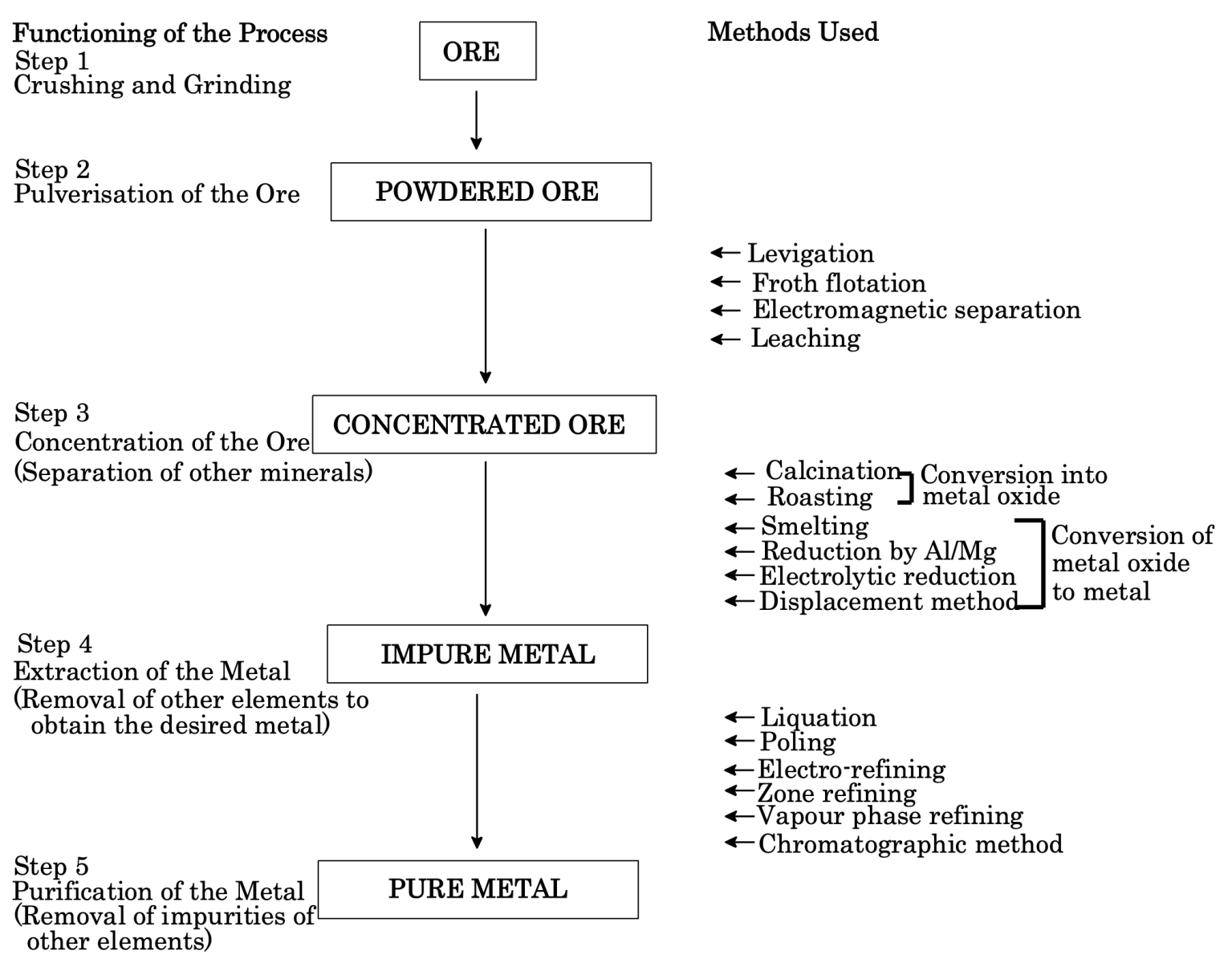
SUMMARY OF METHODS OF EXTRACTION OF SOME COMMON METALS
|
Metals |
Occurrence |
Extraction Method |
Remark |
|
1. Lithium |
Spodumene, LiAlSi2O6; Lepidolite, (Li, Na, K)2 Al2(SiO3)3Fe(OH) |
Electrolysis of fused LiCl/KCl |
Because of high reactivity these are extracted under anhydrous conditions. |
|
2. Sodium |
Rock salt, NaCl; Feldspar, Na3AlSi3O8 |
Electrolysis of fused NaCl/CaCl2 |
|
|
3. Magnesium |
Carnallite, KCl.MgCl2.6H2O; Magnesite, MgCO3 |
Electrolysis of fused MgO or MgCl2/CaCl2 |
Carbon reduction is not possible with alkaline earths since a carbide is formed with them. |
|
4. Calcium |
Limestone, CaCO3; Dolomite MgCO3.CaCO3; Gypsum, CaSO4.2H2O |
Electrolysis of fused CaCl2/CaF2 |
|
|
5. Copper |
Copper pyrites, CuFeS2; Cuprite, Cu2O; Malachite, CuCO3.Cu(OH)2 |
Roasting of sulphide. Initially formed Cu2O reduces Cu2S to Cu |
It is an example of auto-reduction. Sulphuric acid leaching is also employed. |
|
6. Aluminium |
Bauxite, Al2O3.2H2O Cryolite, Na3AlF6; Aluminosilicates |
Electrolysis of Al2O3 dissolved in fused Na3AlF6 or Na3AlCl6 |
A cheap source of electricity is needed in the extraction of Al. |
|
7. Zinc |
Zinc blende or Spharellite, ZnS; Calamine ZnCO3 |
Roasting followed by reduction with carbon |
The metal may be purified by fractional distillation. |
|
8. Lead |
Galena, PbS |
Roasting followed by reduction with carbon |
Magnetic separation is employed as the impurities in this case are magnetic. |
|
9. Tin |
Cassiterite, SnO2 |
Carbon reduction of the oxide |
|
|
10. Silver |
Argentite, Ag2S Horn silver, AgCl |
Sodium cyanide leaching of the sulphide and finally displacement of Ag by Zn |
|
|
11. Gold |
Native, occurs in small amounts in ores of Cu, Ag etc. |
Cyanide leaching, same as in case of Ag |
|
|
12. Chromium |
Chromite, FeO.Cr2O3 |
Si or Al reduction of the oxide (Aluminothermic process) |
1. The flux used in the extraction of iron from haematite in the blast furnace is
(A) silica
(B) limestone
(C) PCl5
(D) calcium phosphate
Sol.(B).
2. Fusion mixture is
(A) K2CO3 + Na2CO3
(B) KHSO4 + NaHSO4
(C) K2CO3 + NaHSO4
(D) KHSO4 + NaSO4
Sol.(A).
3. Which of the following methods is not used in making steel?
(A) Duplex method
(B) Bersem and Thomson
(C) Open hearth
(D) Ostwald
Sol.(D).
4. Corundum is
(A) SrO2
(B) Al2O3
(C) CaCl2
(D) Cu2Cl2
Sol.(B).
5. Chemical reduction is not suitable for converting
(A) bauxite into aluminium
(B) cuprite into copper
(C) haematite into iron
(D) zinc oxide into zinc
Sol.(A).
6. Thomas slag is
(A) Ca3(PO4)2
(B) CaSO4
(C) CaCO3
(D) (NH4)2CO3
Sol.(A).
7. Blister copper is
(A) pure copper
(B) zinc
(C) copper containing some impurity
(D) alloy of copper
Sol.(C).
8. In froth floatation process which of the following is used as a froth, i.e. which helps in the formation of a stable froth?
(A) Potassium xanthate
(B) Pine oil
(C) FeSO4
(D) NaOH
Sol.(B).
9. The process of converting hydrated alumina into anhydrous alumina is called
(A) roasting
(B) calcination
(C) smelting
(D) refining
Sol.(B).