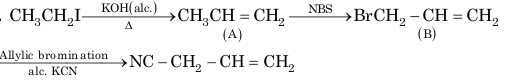Amines
Amines are nitrogen containing organic compounds, which may be considered as derivatives of ammonia in which hydrogen atoms are replaced by alkyl or aryl groups. Amines are classified as primary, secondary or tertiary depending on the number of alkyl groups attached to nitrogen atom.
Primary amine
H₃C—NH₂
Secondary amine
H₃C—NH—CH₃
Tertiary amine
H₃C—N(CH₃)₂
Methods Of Preparation
1. Reduction:
Amines can be prepared by the reduction of alkyl cyanide, nitroalkane and oxime.
(a) Mendius reaction:
The reduction of alkyl cyanides with the reductant (Na + C₂H₅OH), is known as Mendius reaction.
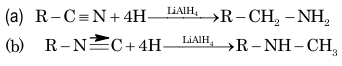
R—C≡N + 4[H] → R—CH₂—NH₂ (Na/C₂H₅OH)
(b) Reduction of nitroalkanes:
Reduction of nitroalkanes by Sn/HCl, Zn/HCl, H₂/Ni or LiAlH₄ gives primary amines.
R—NO₂ + 6[H] → R—NH₂ + 2H₂O
(c) Reduction of oximes:
Reduction of oximes with LiAlH₄ or H₂/Ni gives primary amine.
R—CHO → R—CH=NOH → R—CH₂—NH₂ + H₂O (LiAlH₄/4[H])
2. Hydrolysis:
(a) Hydrolysis of isocyanides
R—N≡C + 2H₂O → R—NH₂ + HCOOH (dilute H₂SO₄)
(b) Hydrolysis of isocyanates
R—N=C=O + 2KOH → R—NH₂ + K₂CO₃
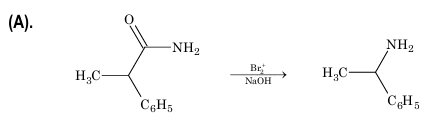
3. Hofmann's bromamide reaction:
Amide is treated with bromine and KOH solution to give primary amine containing one carbon atom less than the amide.
R—CONH₂ + Br₂ + 4KOH → R—NH₂ + K₂CO₃ + 2KBr + 2H₂O
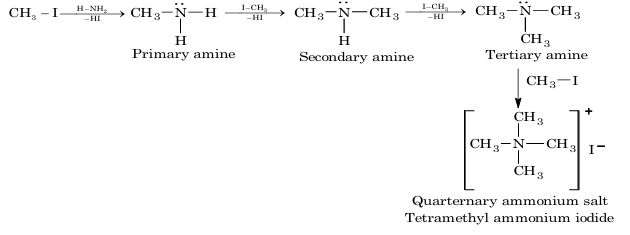
4. Ammonolysis of alkyl halide:
5. From acyl chloride (Curtius reaction):
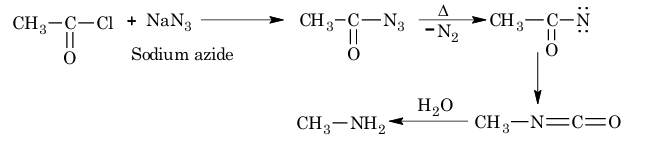
6. From carboxylic acid (Schmidt reaction):
CH₃COOH + N₃H → CH₃NH₂ + CO₂ + N₂ (H₂SO₄)
7. Gabriel phthalimide reaction:
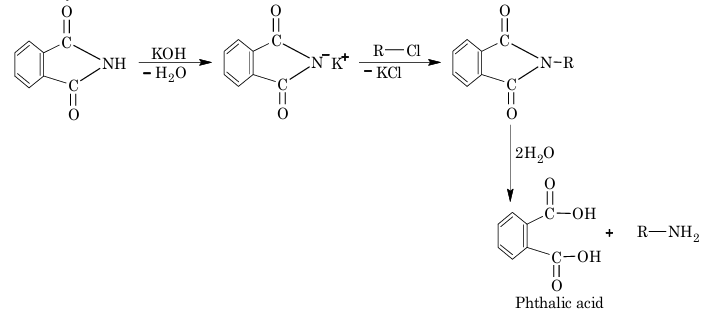
8. From reductive amination of aldehydes and ketones:
(a) From aldehydes:
R—CHO + NH₃ → [R—CH=NH] → R—CH₂—NH₂ (H₂/Ni, -H₂O)
(b) From ketones:
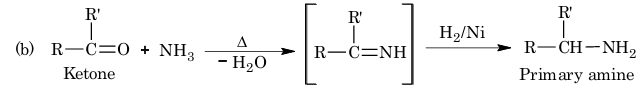
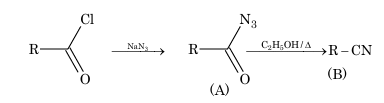
Example 1: The products (A) and (B) formed in the given reaction is
RCOCl + NaN₃ → (A) → (B) (C₂H₅OH/Δ)
(A) RCON and RCN
(B) RCN and RNC
(C) RCON₃ and RCN
(D) RCON₃ and RNCO
Solution: (C)
Example 2: Alkanamide which on Hofmann's reaction gives 1-phenyl ethyl amine is
(A) 2-phenylpropanamide
(B) 3-phenylpropanamide
(C) 2-phenylethanamide
(D) N-phenylethanamide
Solution: (A)
Physical Properties Of Amines
- Boiling points: Primary amines and secondary amines form hydrogen bonding. There are small intermolecular association in primary and secondary amines. Thus, the boiling point is in following order: Primary amine > Secondary amine > Tertiary amine
- Order of volatility: Tertiary amine > Secondary amine > Primary amine
- Solubility: Low molecular weight amines are easily soluble in water. The solubility in water decreases with increasing size of alkyl groups. Amines are capable of forming H–bonding with water. More H–bonding, more solubility in water.
Chemical Reactivity Of Amines
Basic nature of amines: All amines behave as a base because of the presence of a lone pair of electrons on the nitrogen atom.
Aliphatic amines are stronger bases than ammonia. This is due the reason that alkyl groups are electron donating groups. As a result, the electron density on the nitrogen atom increases and thus they can donate the lone pair of electron more easily than ammonia.
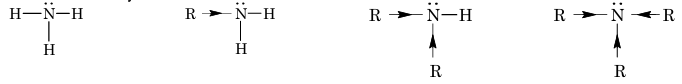
However, in aqueous solution the order of basicity is different:
(a) (CH₃)₂NH > CH₃NH₂ > (CH₃)₃N > NH₃
(b) (C₂H₅)₂NH > C₂H₅NH₂ > NH₃ > (C₂H₅)₃N
(c) (CH₃)₂CNH₂ > NH₃ > [(CH₃)₃CH]NH₂ > [(CH₃)₃CH]₃N
(d) NH₃ > (CH₃)₃CNH₂ > [(CH₃)₃C]₂NH > [(CH₃)₃C]₃N
Reasons of deviation of basicity of amines
(a) Steric–hindrance: In tertiary amines, R₃N, three alkyl groups attached to nitrogen atom are bulkier and as such exert steric hindrance and do not allow the approaching proton to reach near the nitrogen atom.
(b) Decrease in hydration of protonated amine: The stability of the ammonium cation due to H–bonding depends upon the number of hydrogen atoms present on nitrogen atom, more the number of hydrogen atoms more stable is the ammonium cation.
Thus, the ammonium cation derived from primary amine is the most stable as it has three hydrogen atoms which can form hydrogen bonds with water molecule.
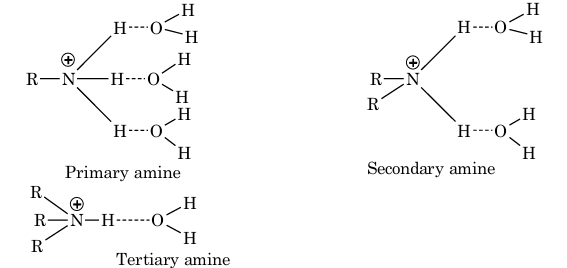
The ammonium cation derived from secondary amine is less stable since it has two hydrogen atoms while tertiary amine is the least stable since it has only one hydrogen atom which can form hydrogen bond with water molecule.
Note: Aniline is less basic than aliphatic amines as the lone pair of electrons present on nitrogen atom interact with the delocalized π-orbital of benzene ring, hence it is less available on nitrogen atom.
The basic character for aniline, pyridine and pyrrole follows the order, (Kb given the parenthesis):
Pyridine > Aniline > Pyrrole
[2.3×10⁻⁹] [4.2×10⁻¹⁰] [2.5×10⁻¹⁴]
Chemical Reactions
1. Alkylation:
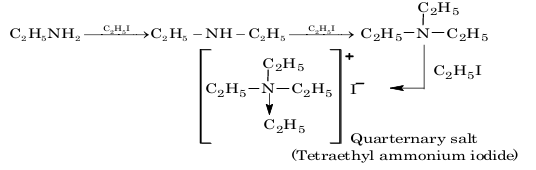
2. Acetylation:
C₂H₅NH₂ + CH₃COCl → CH₃CONHC₂H₅ + HCl (N-ethyl acetamide)
3. Benzoylation (Schotten–Bauman reaction):
C₂H₅NH₂ + C₆H₅COCl → C₆H₅CONHC₂H₅ (N-ethyl benzamide)
4. Hofmann mustard oil reaction:
Only primary amine give pungent mustard oil smell, while secondary amines are not decomposed by HgCl₂ and tertiary amines do not react with CS₂.
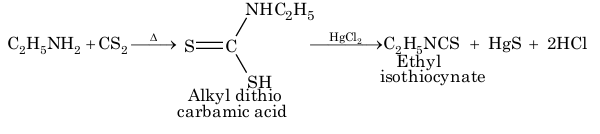
5. With CHCl₃ / KOH (Isocynide test):
C₂H₅NH₂ + CHCl₃ + KOH → C₂H₅N≡C + 3KCl + 3H₂O
6. Formation of Schiff's base:
C₂H₅NH₂ + C₆H₅CHO → C₂H₅N=CHC₆H₅ + H₂O
Distinction between 1°, 2° and 3° amines
1. Reaction with HNO₂
(a) Primary amines react with nitrous acid to produce nitrogen gas.
C₂H₅NH₂ + HONO → C₂H₅OH + N₂ + H₂O
CH₃NH₂ is an exception to this reaction.
CH₃NH₂ + 2HNO₂ → CH₃O + N₂O + N₂ + 2H₂O 2CH₃NH₂ + 2HNO₂ → CH₃OCH₃ + 2N₂ + 3H₂O
(b) Secondary amines react with nitrous acid to produce a yellow oily layer.
(C₂H₅)₂NH + HONO → (C₂H₅)₂N—N=O + H₂O (N-nitroso diethyl amine - yellow oil)
(c) Tertiary amines react with nitrous acid to form soluble nitrite.
(C₂H₅)₃N + HNO₂ → (C₂H₅)₃NHNO₂⁻ (Triethyl ammonium nitrate - soluble)
2. Reaction with benzenesulphonyl chloride
(a) Primary amine reacts with benzenesulphonyl chloride to form a precipitate, which is soluble in NaOH solution.
C₆H₅SO₂Cl + C₂H₅NH₂ → C₆H₅SO₂NHC₂H₅ + HCl (N-ethyl benzene sulphonamide - precipitate)
(b) Secondary amines react with benzenesulphonyl chloride to give a precipitate, which is insoluble in NaOH solution.
C₆H₅SO₂Cl + HN(C₂H₅)₂ → C₆H₅SO₂N(C₂H₅)₂ + HCl (N, N-diethyl benzene sulphonamide - precipitate)
(c) Tertiary amines do not react with benzenesulphonyl chloride as they do not possess replaceable hydrogen atoms.
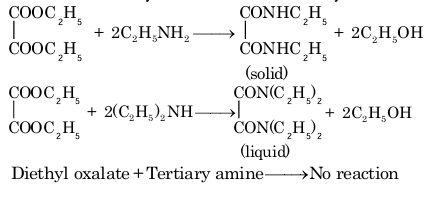
3. Reaction with diethyloxalate (Hofmann's method)
Primary amines react to form solid crystalline oxamide and secondary amines react to give oily dialkyl oxamic ester. Tertiary amines do not react as they do not contain a replaceable H atom.
Benzene Diazonium Chloride
Methods of Preparation
Aromatic diazonium chlorides are generally prepared by adding a cold aqueous solution of sodium nitrite to the solution of a primary aromatic amine in an acid at 273–278 K.
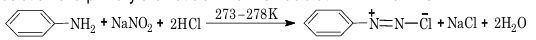
The process of conversion of a primary aromatic amine into its diazonium chloride is called diazotization.
Some Important Chemical Reactions of Benzene Diazonium Chloride
1. Sandmeyer reaction:
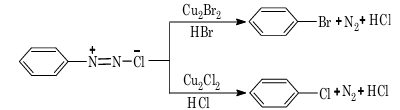
Note: In the above reaction, if copper powder is used in place of cuprous salt, the reaction is known as Gattermann reaction.
2. Balz–Schiemann reaction:

3. Coupling reaction:
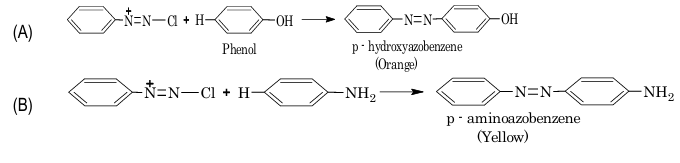
Exercise 2:
Question: Identify the product Z in the series
CH₃CN → X → Y → Z (Na/C₂H₅OH, HNO₂, [O])
(A) CH₃CHO
(B) C₂H₅CONH₂
(C) CH₃COOH
(D) C₂H₅NHOH
Solution: (C). CH₃CN → C₂H₅NH₂ → C₂H₅OH → CH₃COOH
Nitro Compounds
The class of organic compounds containing nitro group as a substituent are called nitro compounds. They may be aliphatic or aromatic depending upon whether the nitro group is attached to an alkyl or an aryl group.
Nitroalkanes are further classified as primary, secondary and tertiary depending upon whether the nitro group is attached to a primary, secondary or a tertiary carbon atom.
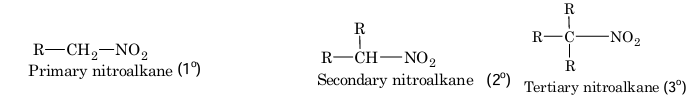
Methods of Preparation
1. From alkyl halides:
R—I + AgNO₂ → R—NO₂ + R—O—N=O (Alcohol-water, Minor/Major)
R—I + KNO₂ → R—O—N=O + R—NO₂ (Alcohol-water, Major/Minor)
2. From tertiary alkyl amines:
(CH₃)₃C—NH₂ + KMnO₄ → (CH₃)₃C—NO₂
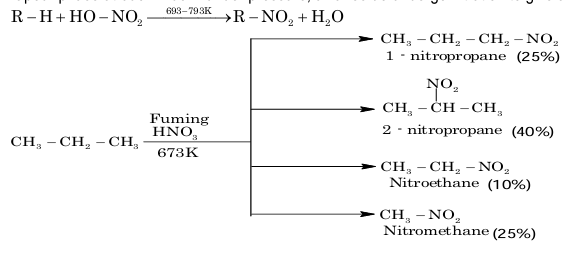
3. From nitration of alkanes:
Alkanes do not undergo nitration easily. However, with fuming HNO₃ in the vapour phase at 693–793 K under pressure, alkanes do undergo nitration to give a mixture of nitroalkanes.
Chemical Properties of Nitro Compounds
1. Reduction:

Note: The final product, however, depends upon the pH of the reaction medium and nature of the reducing agent.
(a) Reduction in the acidic medium:
CH₃—CH₂—NO₂ → CH₃—CH₂—NH₂ (Sn/HCl)
(b) Reduction in the neutral medium:
CH₃—NO₂ → CH₃—NH—OH + ZnO (Zn/NH₄Cl, H₂O) Nitromethane N-Methyl hydroxylamine
(c) Catalytic reduction:
CH₃—CH₂—NO₂ + 3H₂ → CH₃—CH₂—NH₂ + 2H₂O (Pd/C, Ethanol)
(d) Reduction with metal hydrides:
CH₃—CH₂—NO₂ → CH₃—CH₂—NH₂ (LiAlH₄, Ether)
2. Hydrolysis:
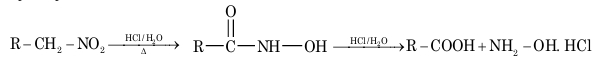
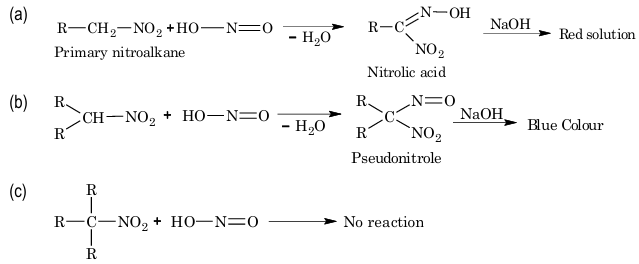
3. Action with nitrous acid:
Primary, secondary and tertiary nitroalkanes behave differently towards nitrous acid.
4. Halogenation:
In this reaction, all the α–hydrogen atoms of nitroalkanes are successively replaced by the halogen atoms.
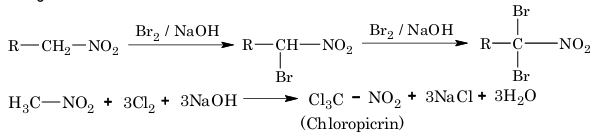
Example 3: In which of the following sequence of reactions the end product does not exhibit tautomerism?
(A) CH₃CH₂NH₂ → AgNO₂ → NOCl
(B) (CH₃)₂CHNH₂ → AgNO₂ → NOCl
(C) (CH₃)₃CNH₂ → AgNO₂ → NOCl
(D) CH₃CH(NH₂)C₆H₅ → AgNO₂ → NOCl
Solution: (C) In reaction sequence (C), the end product is a 3° nitro compound.
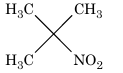
It does not have α–hydrogen, hence tautomerism is not possible.
Cyanide And Isocyanides
The class of organic compounds obtained by replacement of the H–atom of hydrogen cyanide by an alkyl or aryl group is called cyanides or nitriles. The general formula is, R—C≡N or Ar—C≡N, (where R is an alkyl group and Ar is an aryl group).
When H–atom of hydrogen isocyanide is replaced by alkyl or the aryl group, is called isocyanides or isonitriles. It is also called carbylamines. The general formula is, R—N≡C or Ar—N≡C
Note: C≡N group can be attached to the alkyl or the aryl group either through the carbon atom or through the nitrogen atom. Such a group which can be linked through two different sites, is called an ambidient group.
Methods of Preparation
1. From alkyl halide:
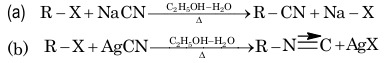
2. From acid amides:
CH₃—CO—NH₂ → CH₃—C≡N + H₂O (P₂O₅)
3. From aldoximes:
CH₃—CH=NOH → CH₃—C≡N + H₂O ((CH₃CO)₂O)
4. From primary amine:
R—NH₂ + CHCl₃ + 3KOH → R—N≡C + 3KCl + 3H₂O (alc.)
Chemical Properties of Cyanides And Isocyanides
1. Hydrolysis:
(i) Complete hydrolysis:
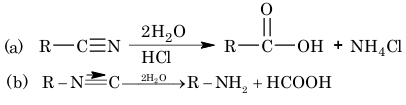
(ii) Partial hydrolysis:

2. Reduction:
3. Stephen's reduction:

4. Reaction with Grignard reagent:
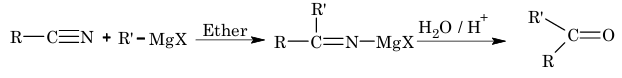
5. Addition reaction of isocyanides:
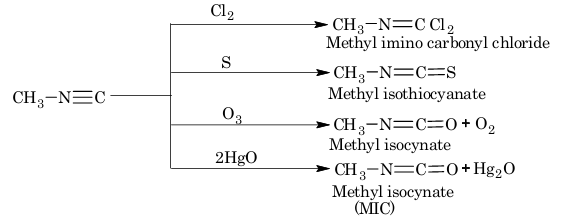
Note: The methylisocyanate (MIC) gas was responsible for the Bhopal gas tragedy in December, 1984.
Example 4: Identify C in the series
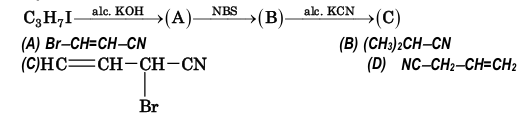
Solution: (D)