Carboxylic Acid & ITs Derivatives: Carboxylic acids are organic compounds which contain carboxylic group -COOH.
The name carboxyl is derived from two words, i.e. carbonyl and hydroxyl because in carboxylic acid both carbonyl and hydroxyl groups are directly linked to each other.
Carboxylic acids are classified in accordance to the number of –COOH groups present in the molecule.
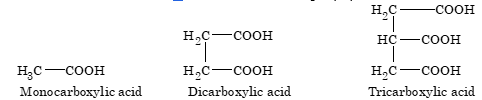
Carboxylic acids are further classified as aliphatic and aromatic. In aromatic, the carboxylic group is attached to aryl group.
Monocarboxylic acid of aliphatic species are commonly known as fatty acid, containing more than twelve carbon atoms, obtained from fats.
General Methods of Preparation
From Alcohols
Oxidation of Alcohol: Primary alcohols on oxidation forms monocarboxylic acid with same number of carbon atoms. The oxidation can be carried out in presence of potassium dichromate and dilute sulphuric acid. The initial product of oxidation is the corresponding aldehyde which then forms acid.
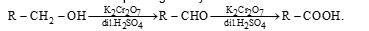
In case alkaline KMnO₄ is used as an oxidizing agent, the aldehyde undergoes oxidation more rapidly than primary alcohol.
The selective oxidation is possible in presence of CrO₃ and H₂SO₄ in acetone. It does not affect C=C and >C=C< but selectively oxidizes hydroxyl group to COOH.
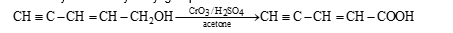
- From Alkene
(i) Oxidative cleavage: Alkene can be oxidized into corresponding carboxylic acid with hot alkaline KMnO₄.
CH₂=CH₂ + alkaline KMnO₄ → 2HCOOH
CH₃CH=CHCH₃ + KMnO₄ + H⁺ → CH₃COOH + CH₃COOH
(ii) Carbonylation: (Koch reaction): When a mixture of alkene, carbon monoxide and steam is heated under pressure at 350°C in presence of phosphoric acid, monocarboxylic acid is formed.
CH₂=CH₂ + CO + H₂O → CH₃CH₂COOH
- From Alkyne
CH≡CH + KMnO₄ + H₃O⁺ + H₂O → 2HCOOH
R–C≡C–R' + 3[O] + H₃O⁺ → RCOOH + R'COOH
On oxidation with chromic acid (K₂CrO₄ + H₂SO₄) single carboxylic acid is formed
CH≡CH + H₂O + [O] → CH₃COOH (chromic acid)
CH₃–C≡CH + H₂O + [O] → CH₃CH₂COOH
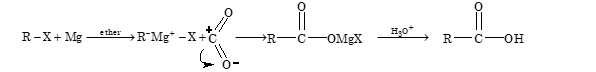
By Carbonation of Grignard Reagents
Grignard reagent on treatment with carbon dioxide forms a addition product, which on hydrolysis gives acid.
Hydrolysis of Trihalogen Derivatives
Trihalogen derivatives of alkanes in which all the three halogen atoms are attached to same carbon atom, on hydrolysis with aqueous alkali solution, form mono carboxylic acids
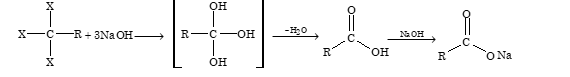
Heating Sodium Alkoxide with Carbon Monoxide
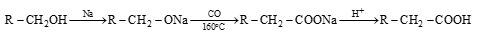
Hydrolysis of Acid Derivatives
- (i) From ester:
R–COOR' + H–OH → R–COOH + R'–OH
- (ii) From acid amide:
R–CONH₂ + H–OH → R–COOH + NH₃ (alkali or acid)
- (iii) From anhydrides:
(RCO)₂O + H–OH → 2R–COOH
- (iv) From acid halide:
R–COX + H–OH → R–COOH + H–X
By Hydrolysis of Alkane Nitrile
The compound containing CN group can be hydrolysed to carboxylic acid with an additional carbon atom than original alkyl halide.
![]()
Exercise 1: The most appropriate reagent for the conversion of 2-pentanone to butanoic acid is
(A) NaOI; H₃O⁺
(B) KMnO₄, NaOH, H₃O⁺
(C) K₂Cr₂O₇, H₂SO₄
(D) AgOH, NH₄OH; H₃O⁺
Illustration 1: On reaction with dry ice ethyl magnesium bromide followed by hydrolysis gives
(A) methanoic acid (B) ethanoic acid (C) propanoic acid (D) methane
Solution: (C)
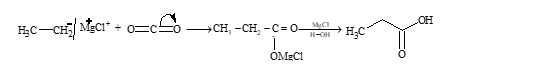
PHYSICAL PROPERTIES
- The lower fatty acids upto C₁₀ are colourless liquids. The higher ones are waxy solids.
- The molecules of carboxylic acid are polar in nature and exhibit hydrogen bonding.
- The lower members are highly soluble in water but the solubility decreases with the rise of molecular mass. The acids above C₁₀ carbon are insoluble in water. It is due to its polar nature and tendency to form H-bond.
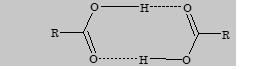
- Boiling point of carboxylic acids increase regularly with increase in molecular mass. Boiling points of acid are higher than alcohol and ether. This is due to intermolecular hydrogen bonding between acid molecules. It exists in dimeric form
CHEMICAL PROPERTIES
The reactions of carboxylic acid can be studied under four different categories:
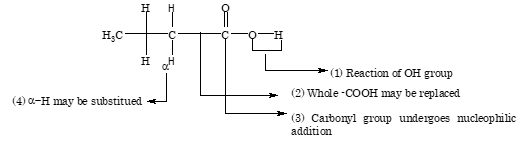
Reaction involving Alkyl Group
α-hydrogen atom undergoes substitution due to presence of electronegative carbonyl group
α-halogenation of acid (Hell Volhard Zelinski Reaction):
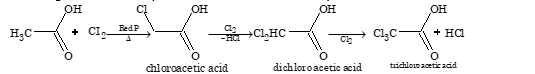
Direct iodination can be done in the presence of an oxidizing agent like iodic acid or mercuric oxide.

Note: Tertiary acids show no substitution due to absence of α-hydrogen
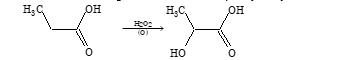
Oxidation
Monocarboxylic acids except formic acid are resistant to oxidation. But at higher temperature it oxidizes into carbon dioxide and water.
HCOOH + ½O₂ → CO₂ + H₂O
In presence of H₂O₂ and Ag₂O, it is oxidized to α-hydroxy acid.
Reaction Involving Carboxyl Group
- Salt formation:
Carboxylic acids are weak acids and their carboxylate ions are strong conjugated bases. They are slightly alkaline due to hydrolysis of carboxylate ions. The order of acidity and basicity of corresponding conjugated bases are follows:
Acidity:RCOOH > HOH > R-OH > CH≡CH > NH₃ > RH
Basicity:R⁻COO⁻ < OH⁻ < RO⁻ < CH≡C⁻ < NH₂⁻ < R⁻
Carboxylic acids react with metal to liberate hydrogen. It is soluble in both NaOH and NaHCO₃ solution.
2CH₃COOH + 2Na → 2CH₃COONa + H₂
CH₃COOH + NaOH → CH₃COONa + H₂O
CH₃COOH + NaHCO₃ → CH₃COONa + H₂O + CO₂
Acidity of carboxylic acids
The strength of an acid depends on the ability of acid to lose proton. When dissolved in water it is expressed in terms of dissociation constant (Kₐ)
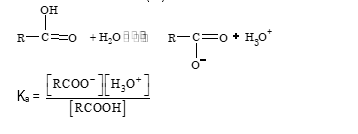
Acid strength ∝ Kₐ value ∝ 1/pKₐ value
- Cause of acidic nature:
The acidity of carboxylic acid is due to resonance stabilization of its anion.
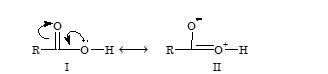
Due to electron deficiency on oxygen atom of the hydroxyl group (structure – II), there is displacement of electron pair of O–H bond towards oxygen atom. This help in release of hydrogen as proton. The resulting carboxylate ion is also stabilized by resonance as resonance energy of the carboxylate ion is much higher than that of undissociated acid.
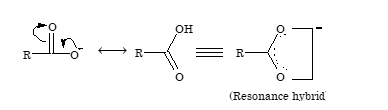
-
Effect of substitution on acidity
- Acidic strength ∝ -I effect (electron withdrawing group)
- Acidic strength ∝ 1/+I effect
- Increase in distance of halogen atoms from COOH group decreases acidity.
- Increase in electronegativity of halogen atoms increases the acidity.
-
Effect of electron withdrawing groups (-I effect):
(i) An electron withdrawing group stabilizes the anion by dispersing the negative charge and therefore increases acidity.
Cl₃C–COOH > Cl₂CH–COOH > Cl-CH₂COOH > CH₃COOH
(ii) CH₃CH₂CHClCOOH > CH₃CHClCH₂COOH > ClCH₂CH₂CH₂COOH
(iii) F–CH₂COOH > BrCH₂COOH > ICH₂COOH
-
Effect of electron releasing group (+I effect):
Electron releasing group increase electron density on oxygen atom resulting decrease in stability and decrease acidic strength.
H–COOH > C₆H₅–COOH > CH₃–COOH > (CH₃)₂CH–COOH > (CH₃)₃C–COOH
Exercise 2: Consider the following compounds with regard to their acidities.
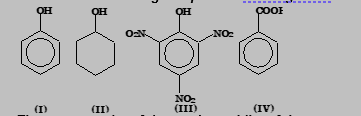
The correct order of decreasing acidity of these compounds is
(A) IV > III > I > II
(B) III > IV > I > II
(C) IV > III > II > I
(D) II > I > III > IV
Illustration 2: Among the compounds
I. CH₃COOH II. CH₃CH₂COOH III. CH₂=CHCOOH IV. HC≡C-COOH
the correct order of decreasing acidity is
(A) I > II > III > IV
(B) IV > III > II > I
(C) IV > III > I > II
(D) III > IV > I > II
Solution: (C) More the 's' character of the C adjacent to COOH group, more will be the electronegativity of that carbon and hence more will be the acidity.
- Reaction with ketene
R–COOH + CH₂=C=O → R–CO-O-CO-CH₃ (Anhydride)
- Reaction with diazomethane
R–COOH + CH₂N₂ → R–COOCH₃ + N₂
Reactions Involving Replacement of OH
-
Formation of acid halide
CH₃COOH + PCl₅ → CH₃COCl + HCl + POCl₃
3CH₃COOH + PCl₃ → 3CH₃COCl + H₃PO₃
CH₃COOH + SOCl₂ → CH₃COCl + SO₂ + HCl
- Reaction with alcohol (esterification)
CH₃COOH + CH₃OH ⇌ CH₃COOCH₃ + H₂O (H₂SO₄)
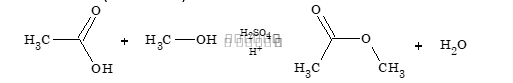
This reaction is reversible and reaches equilibrium when there are enough amount of reactant & product.
Exercise 3 - The rate of acid catalysed esterification of the carboxylic acids
I. CH₃COOH II. CH₃CH₂CH₂COOH III. (CH₃)₂CCOOH IV. C₆H₅CCOOH
with methanol decreases in the order
(A) I > II > III > IV
(B) IV > III > II > I
(C) III > I > II > IV
(D) II > III > IV > I
- Reaction with ammonia
CH₃COOH + NH₃ → CH₃COONH₄ → CH₃CONH₂
(Ammonium acetate → Acetamide)
- Reaction with P₂O₅
2CH₃COOH + P₂O₅ → (CH₃CO)₂O + H₂O (anhydride)
- Reduction
The COOH group is reduced to CH₂OH group in presence of LiAlH₄.
With LiAlH₄ the –COOH is reduced to –CH₂–OH.
On heating with HI & red P, product is alkane
Catalytic reduction gives alkane
R–COOH + LiAlH₄ → R–CH₂–OH + H₂O
In presence of red phosphorus at 200°C, COOH group is reduced to alkane.
R–COOH + 6HI + red P → R–CH₃ + 2H₂O + 3I₂ (200°C)
The product is again alkane when COOH group is catalytically reduced in presence of Ni.
R–COOH + 3H₂ → R–CH₃ + 2H₂O (Ni)
Illustration 3: Propanoic acid → PCl₅ → X → C₆H₆/AlCl₃ → Y
In above sequence Y is
(A) phenyl ethyl ketone (B) phenyl acetate (C) ethyl benzene (D) ethyl benzoate
Solution: (A)
CH₃–CH₂–COOH → CH₃–CH₂–COCl → C₆H₅–CO–CH₂–CH₃
(Friedel Craft reaction)
Reaction Involving COOH as a Whole
- Decarboxylation
It is a process of elimination of CO₂ from a salt of carboxylic acid, in presence of soda lime and the product is alkane.
R–COONa + NaOH/CaO → R–H + Na₂CO₃
Dibasic acids with two –COOH groups on same carbon atom will loose a molecule of CO₂ on simple heating.
R–C(COOH)₂ → R–COOH + CO₂
Decarboxylation of β-keto acids will take place with warm dil. H₂SO₄ to form aldehyde.
CH₃–CO–CH₂–COOH → CH₃–CO–CH₃ + CO₂ (dil. H₂SO₄)
- Hunsdiecker reaction
An alkyl halide is formed when the silver salt of carboxylic is heated with halogen
CH₃–COOAg + Br₂ → CH₃–Br + AgBr + CO₂ (CCl₄)
A carboxyl radical is produced in a two-step process:
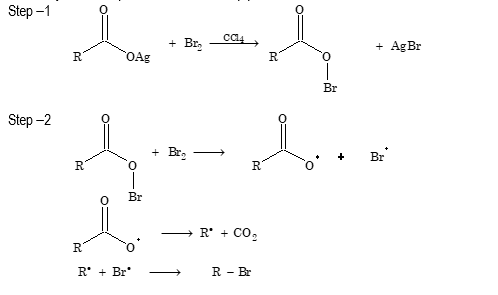
- Formation of amine (Schmidt reaction)
Primary amine containing one carbon atom less than the acid are formed when monocarboxylic acid react with hydrazoic acid (N₃H) in presence of concentration H₂SO₄.
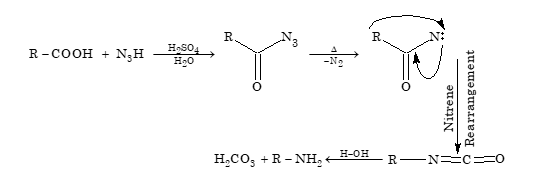
-
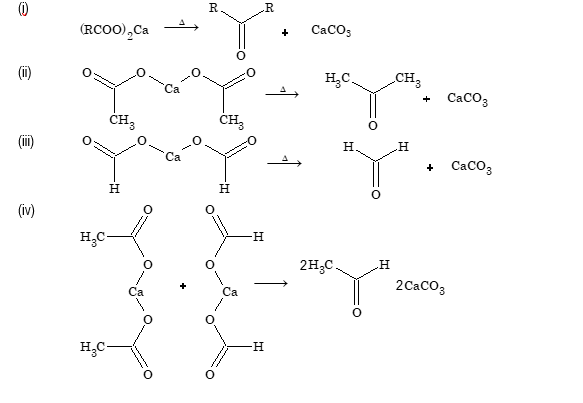
Dry distillation of acid (Heating of calcium salt of acid)
When calcium salt of fatty acids other than calcium formate are heated, ketones are formed. When calcium formate is heated, formaldehyde is formed. When a mixture of calcium salt of a fatty acids and calcium formate is heated, aldehyde is formed.
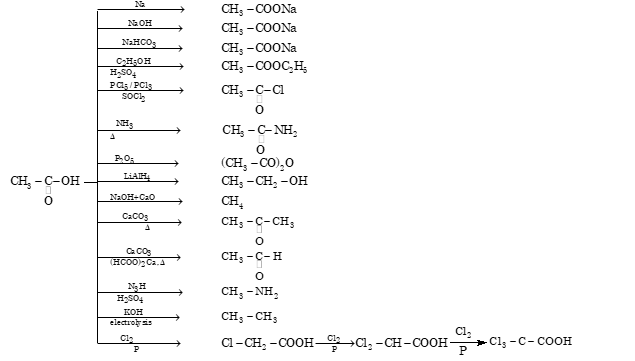
Reaction Chart of Acetic Acid
Identification Test
| Test | Acetic acid | Formic acid |
|---|---|---|
| 1. Tollens reagent | Unaffected | Gives silver mirror or black ppt. HCOOH + Ag₂O → 2Ag + CO₂ + H₂O |
| 2. Fehling solution | Unaffected | Gives red ppt. HCOOH + 2CuO → Cu₂O + CO₂ + H₃O |
| 3. Mercuric chloride | Unaffected | Form white ppt. which change to Greyish black. HgCl₂ → Hg₂Cl₂ → 2Hg |
| 4. Acidic KMnO₄ | Unaffected | Decolourize |
| 5. Acid + NaHSO₃ + Sodium nitroprusside | Unaffected | Greenish blue colour |
| 6. Acid + FeCl₃ | Wine red | Red colour which changes to brown ppt. on heating |
Exercise 4 - Consider the following sequence of reaction
CHO-CHO + HCN (excess) + H₂O → A → B (heat)
The product (B) is
(A) lactic acid
(B) oxalic acid
(C) tartaric acid
(D) succinic acid
Illustration 4: Which of the following reactions does not involve decarboxylation?
(A) CH₃COOAg + Br₂ → (Heat, CCl₄)
(B) CH₃COCH₂CH₃COOH + Br₂ → (Heat)
(C) CH₃COO⁻ → (anodic oxidation)
(D) CH₃CH₂COOH + Br₂ + P → (Heat)
Solution: (D) In (D), the hydrogen will be substituted by Br.
ACID DERIVATIVES
The classes of compounds obtained from acids by the replacement of –OH group of –COOH group with –X, -NH₂, -OOCR or –OR are termed as acid derivatives
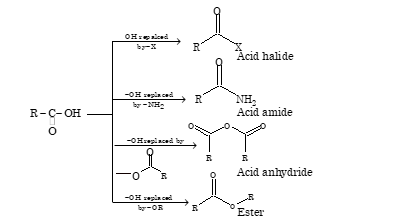
Acyl derivatives are characterized by nucleophilic substitution reactions.
R-CO-L + Nu⁻ → R-CO-Nu + L⁻
(L = leaving group)
ACETYL CHLORIDE
- Method of Preparation
Acetyl chloride is prepared by heating acetic acid with phosphorous trichloride, phosphorus pentachloride or thionyl chloride. Thionyl chloride is the best reagent because the byproducts are in gaseous forms, which are easily separated.
(i) CH₃COOH + PCl₅ → CH₃COCl + HCl + POCl₃
(ii) 3CH₃COOH + PCl₃ → 3CH₃COCl + H₃PO₃
(iii) CH₃COOH + SOCl₂ → CH₃COCl + SO₂ + HCl
- Physical Properties
- It is a colourless liquid with a pungent odour.
- It is soluble in ether acetone and acetic acid.
- Chemical Properties
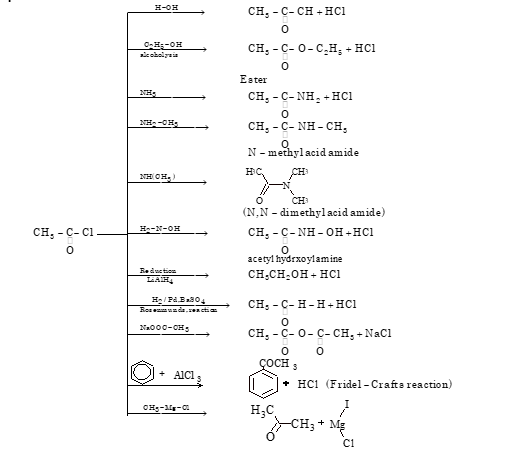
ACETIC ANHYDRIDE
- Methods of Preparation
1. It is prepared by heating glacial acetic acid with a dehydrating agent like P₂O₅ or anhydrous ZnCl₂.
2CH₃COOH + P₂O₅ → (CH₃CO)₂O + H₂O
2. Acetic anhydride is prepared by heating sodium salt of acid with acid halide.
CH₃COONa + CH₃COCl → (CH₃CO)₂O + NaCl
-
Physical Properties
- It is colourless, pungent smelling liquid. It boils at 139.5°C.
- It is sparingly soluble in water but soluble in ether, alcohol and acetic acid.
- Chemical Properties
The reactivity of anhydride is less than acid halide. One half of the acetic anhydride is used for acetylation while other half is converted into acetic acid.
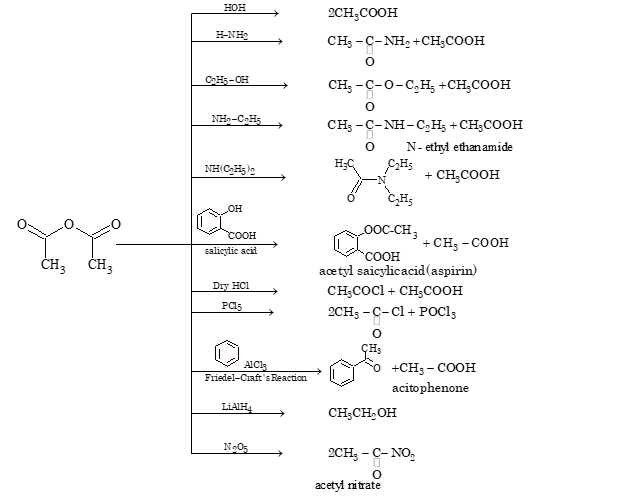
Illustration 5: The formula C₄H₄O₄ can represent
(A) A cyclic ester of dibasic acid
(B) A cis–dibasic acid
(C) A Trans dibasic acid
(D) All are correct
Solution: (D)
(A) Cyclic anhydride structure
(B) cis-butenedioic acid (maleic acid)
(C) trans-butenedioic acid (fumaric acid)
Exercise 5 Formyl chloride on reaction with hydrogen in presence of Pd/BaSO₄ gives
(A) Formaldehyde
(B) CO + HCl
(C) Methanol
(D) None
Exercise 6: Benzyl chloride can be prepared by treating benzoic acid with
(A) SO₂Cl₂
(B) SOCl₂
(C) Cl₂, hν
(D) Cl₂, H₂O
Illustration 6: Which of the following reagents is able to convert an alcohol into an alkyl chloride but is not able to convert a carboxylic acid into an acid chloride?
(A) PCl₃ (B) PCl₅ (C) SOCl₂ (D) HCl, ZnCl₂
Solution: (D)
ACETAMIDE
- Methods of Preparation
(i) Laboratory preparation:
Acetic acid on reaction with ammonia gives ammonium acetate, which on heating gives acetamide.
CH₃COOH + NH₃ → CH₃COONH₄ → CH₃CONH₂ + H₂O
(ii) By ammonolysis:
All acid derivatives on ammonolysis gives acetamide.
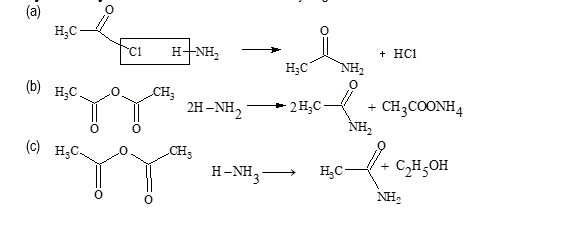
(iii) By partial hydrolysis of cyanide:
Alkyl cyanide on hydrolysis in presence of alkaline H₂O₂ or by dil. H₂SO₄ gives acetamide.
CH₃CN + H₂O₂ (alkaline) + H₂O → CH₃CONH₂
- Physical Properties
- Acetamide is a colourless crystalline solid, it is readily soluble in water and alcohol.
- It exist as a dimer due to hydrogen bonding
Chemical Properties
- (i) Hydrolysis:
Acetamide is hydrolysed by water, rapidly by acids and more rapidly by alkalies.
CH₃CONH₂ + H₂O → CH₃COOH + NH₃
CH₃CONH₂ + NaOH → CH₃COONa + NH₃
(iii) By partial hydrolysis of cyanide:
Acetamide behaves as an amphoteric compound i.e. it shows acidic as well as basic nature. It forms salt with acids and bases both.
CH₃CONH₂ + HCl(conc.) → CH₃CONH₂.HCl (Acetamide hydrochloride)
CH₃CONH₂ + Na (ether) → CH₃CONHNa + ½H₂ (Sodium acetamide)
(ii) Amphoteric nature:
Acetamide on reduction in presence of sodium and ethanol or ethereal solution of LiAlH₄ gives alkyl amine.
CH₃CONH₂ + 4H → CH₃CH₂NH₂ + H₂O (ether)
(iii) Dehydration:
When heated with P₂O₅ or POCl₃, it forms alkyl cyanide.
CH₃CONH₂ + P₂O₅ → CH₃CN + H₂O
(iv) Action of nitrous acid:
CH₃CONH₂ + H-NO₂ → CH₃COOH + N₂ + H₂O
(v) Action with PCl₅:
When heated with PCl₅, acetamide forms methyl cyanide
CH₃CONH₂ + PCl₅ → CH₃CN + POCl₃ + 2HCl
(vi) Hofmann bromide reaction or Hofmann degradation:
Amides when heated with bromine and caustic soda, yield primary amines containing one carbon atom less than amide.
CH₃CONH₂ + Br₂ + KOH → CH₃NH₂
ANSWER TO EXERCISES
| Exercise | Answer |
|---|---|
| Exercise 1 | A |
| Exercise 2 | B |
| Exercise 3 | A |
| Exercise 4 | C |
| Exercise 5 | B |
| Exercise 6 | B |