An inorganic salt is made up of two parts, i.e. cation (basic radical) and anion (acidic radical). The detection and identification of cations and anions in an inorganic salt or mixture of salts is known as qualitative analysis.
PRELIMINARY TESTS
Physical Appearance
It involves the examination of colour, smell, density etc. The salts of some transition elements have characteristic colours as mentioned in Table.
| Radicals | Characteristic Colours |
|---|---|
| Cu2+, Ni2+ | Blue or bluish green |
| Fe2+ | Light green |
| Cr3+ | Dark green |
| Fe3+ | Yellowish brown |
| Co2+ | Pink violet |
| Mn2+ | Light pink |
Dry Heating Test
It involves heating a small amount of the given sample of salt or mixture in a dry test tube. The identification can be done on the basis of below mentioned observations.
Gas Evolved
- Carbonates and some bicarbonates give CO2
CaCO3 → CaO + CO2↑
- Sulphates give SO2
SnSO4 → SnO2 + SO2↑
ZnSO4 → ZnO + SO2↑ - Hydrated chlorides give HCl
ZnCl2·6H2O → Zn(OH)Cl + HCl↑ + 5H2O
- Hydrated sulphides give H2S
BaS·2H2O → Ba(OH)2 + H2S↑
- Some nitrates give NO2
2Pb(NO3)2 → 2PbO + 4NO2↑ + O2↑
- Oxalates give mixture of (CO+CO2)
CaC2O4 → CaO + CO + CO2↑
Residues:
- Zinc salts give yellow residue when hot but changes to white when it is cold.
ZnCO3 → ZnO + CO2↑ (Residue)
- Hydrated copper sulphate gives white residue.
CuSO4·5H2O → CuSO4 + 5H2O
(Blue) → (White)
Flame Test
This test is performed with the paste of the given salt / mixture in conc. HCl. The paste is taken on a platinum wire and is heated in an oxidizing flame. The observation is mentioned in Table 2.
| Cation | Colour of flame |
|---|---|
| Ca2+ | Brick red |
| Sr2+ | Crimson red |
| Ba2+ | Grassy green |
| Cu2+ | Bluish |
| K+ | Pale violet |
| Na+ | Golden yellow |
| Zn2+, Mn2+ | Green flashes |
Borax Bead Test
This test is applicable to only coloured salts. This is based on the fact that coloured cations form metaborates of specific colour.
Na2B4O7·10H2O → Na2B4O7 + 10H2O (Borax)
Na2B4O7 → 2NaBO2 + B2O3 (Glassy mass)
- Cr3+ forms green coloured metaborates.
Cr2(SO4)3 + 3B2O3 → 2Cr(BO2)3 + 3SO3 (Green)
- Co2+ ions form blue coloured bead.
CoSO4 + B2O3 → Co(BO2)2 + SO3 (Blue)
- Fe3+ ions form yellow coloured bead.
Fe2O3 + 3B2O3 → 3Fe(BO2)3 (Yellow)
Some metallic ions form different beads in oxidizing and reducing flames.
CuSO4 + B2O3 → Cu(BO2)2 + SO3 (Green - oxidizing flame)
Cu(BO2)2 + C → 2CuBO2 + B2O3 + CO (Colourless - reducing flame)
- Illustration 1: The gas that turns lime water milky is
(A) CO2
(B) SO2
(C) Both of the above
(D) None of the above
Solution: (C). Both CO2 and SO2 turns lime water [Ca(OH)2] milky due to formation of CaCO3 and CaSO3 respectively.
- Illustration 2: Composition of borax bead is
(A) B2O3 (B) Na2BO3 (C) Na2B4O7 (D) B2O3+NaBO2
Solution: (D). B2O3+NaBO2
- Illustration 3: Which compound is formed in borax bead test?
(A) Orthoborate
(B) Metaborate
(C) Double oxide
(D) Tetraborate
Solution: (B). Metaborate
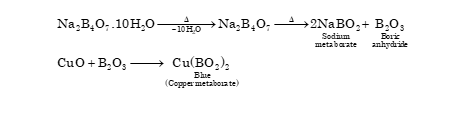
Exercise 1
1) Flame test is not given by
(A) Be2+ (B) Ba2+ (C) Ca2+ (D) None of the above
2) Which of the following salt is colourless?
(A) CdCl2 (B) CuSO4·5H2O (C) MnSO4·7H2O (D) NiSO4·7H2O
3) In a mixture having nitrite and nitrate, nitrite can be destroyed by heating with
(A) Na2CO3 (B) urea (C) oxalic acid (D) NaCl
Exercise 2
A dark green bead in borax bead test indicates the presence of
(A) Cr3+ (B) Mn2+ (C) Co2+ (D) Ni2+
- Illustration 4: When copper (II) nitrate is strongly heated in a dry test tube, it is converted into
(A) Cu metal
(B) cupric oxide
(C) cuprous oxide
(D) cupric nitrate
Solution: (B). 2Cu(NO3)2 → 2CuO + 4NO2↑ + O2↑
IDENTIFICATION OF ACIDIC RADICALS
Dilute Acid Test
This test involves treatment of a given salt/mixture with dil. HCl or dil. H2SO4. It gives the indication of CO32–, SO32–, S2O32–, S2– and NO2– radicals.
Carbonate (CO32–) Gives colourless, odourless CO2 gas which turns lime water milky.
Na2CO3 + 2HCl → 2NaCl + CO2↑ + H2O
Ca(OH)2 + CO2 → CaCO3 + H2O (Lime water - Milkiness)
Sulphite (SO32–). Sulphites decompose to give colourless gas SO2 with smell of burning sulphur which turns K2Cr2O7 paper green.
Na2SO3 + H2SO4 → Na2SO4 + SO2↑ + H2O
K2Cr2O7 + H2SO4 + 3SO2 → Cr2(SO4)3 + K2SO4 + H2O (Green)
Thiosulphate (S2O32–) Reacts with dilute acids to produce SO2 gas along with the formation of yellow turbidity of sulphur.
Na2S2O3 + 2HCl → 2NaCl + SO2↑ + S↓ + H2O
Na2S2O3 + 2AgNO3 → Ag2S2O3 + 2NaNO3 (White)
Ag2S2O3 + H2O → Ag2S + H2SO4 (Black ppt.)
Sulphide (S2–) Sulphides are decomposed by dilute acids to give colourless gas (H2S) with rotten egg's smell.
Na2S + 2HCl → 2NaCl + H2S↑
(CH3COO)2Pb + H2S → PbS + 2CH3COOH (Black ppt.)
Na2S + Na4[Fe(CN)5NO] → Na4[Fe(CN)5NOS] (Sodium nitroprusside - Sodium sulphonitroprusside (violet))
Nitrite (NO2–) Nitrites react with dilute acids to give NO, a colourless gas which turns brown due to oxidation by air.
2NaNO2 + H2SO4 → Na2SO4 + 2HNO2
2HNO2 → NO↑ + H2O + NO2↑
2NO + O2 → 2NO2
FeSO4 + NO → FeSO4·NO or [Fe(H2O)5(NO)]SO4 (Brown ring)
Concentrated H2SO4 Test
This test involves heating of a given salt / mixture with conc. H2SO4. The test gives indication of Cl–, Br–, I–, NO3–, C2O42– radicals.
- Chloride (Cl–) Chlorides react with conc. H2SO4 to give colourless gas with pungent smell (HCl), which gives white dense fumes with NH4OH and white ppt. when passed through silver nitrate solution which is soluble in ammonia.
NaCl + H2SO4 → NaHSO4 + HCl↑
NH3 + HCl → NH4Cl (Fumes)
AgNO3 + HCl → AgCl + HNO3
AgCl + 2NH4OH → [Ag(NH3)2]Cl + 2H2O
- Chromyl chloride test: Chloride salt + K2Cr2O7 + conc. H2SO4 → Red vapours
4KCl + 6H2SO4 + K2Cr2O7 → 6KHSO4 + 2CrO2Cl2 + 3H2O (Red vapours)
CrO2Cl2 + 2NaOH → Na2CrO4 + 2NaCl + 2H2O (Yellow solution)
Na2CrO4 + (CH3COO)2Pb → PbCrO4 + 2CH3COONa (Yellow ppt.)
- Nitrate (NO3–) Nitrates are decomposed by conc. H2SO4 to form brown gas (NO2).
NaNO3 + H2SO4 → NaHSO4 + HNO3
4HNO3 → 4NO2↑ + 2H2O + O2↑
- Ring test: In salt solution add freshly prepared FeSO4 solution. Then add conc. H2SO4 along the sides of the test tube. A dark brown ring is formed at the junction of the two layers.
KNO3 + H2SO4 → KHSO4 + HNO3
6FeSO4 + 3H2SO4 + 2HNO3 → 3Fe2(SO4)3 + 4H2O + 2NO
FeSO4 + NO → FeSO4·NO (Brown ring - Nitrosoferrous sulphate)
The brown ring may also have composition [Fe(H2O)5NO]2+.
- Oxalate (C2O42–) On heating oxalates with conc. H2SO4, CO2 and CO gases are produced.
Na2C2O4 + H2SO4 → H2C2O4 + Na2SO4
H2C2O4 → CO2↑ + CO↑ + H2O
CO2 + Ca(OH)2 → CaCO3 + H2O (Lime water)
2KMnO4 + 3H2SO4 + H2C2O4 → K2SO4 + 2MnSO4 + 4H2O + 10CO2
- Bromide (Br–) Bromides are decomposed by conc. H2SO4 to give reddish brown vapours of Br2, which turn starch paper yellow.
KBr + H2SO4 → KHSO4 + HBr
2HBr + H2SO4 → Br2↑ + SO2↑ + 2H2O
Br2 + Starch → Yellow colour
- Iodide (I-): Iodides react with conc. H2SO4 to give violet vapours of iodine, which turn starch paper blue.
KI + H2SO4 → KHSO4 + HI
2HI + H2SO4 → SO2↑ + I2↑ + 2H2O
I2 + Starch → Blue
- Sulphate (SO42–): Salt solution + Lead acetate → White ppt. (soluble in ammonium acetate)
Na2SO4 + (CH3COO)2Pb → PbSO4 + 2CH3COONa
PbSO4 + 2CH3COONH4 → (CH3COO)2Pb + (NH4)2SO4 (soluble)
- Borate (BO33–): Take salt/mixture in a china dish. Add conc. H2SO4 and alcohol. Ignite the vapours. A green edged flame is obtained.
Na2B4O7 + H2SO4 + 4H2O → 4H3BO3 + Na2SO4
H3BO3 + 3C2H5OH → (C2H5)3BO3 + 3H2O (Ethyl borate - burns with green flame)
Illustration 5:
Reaction of K2Cr2O7 with NaCl and conc. H2SO4 gives
(A) CrCl3 (B) CrOCl2 (C) CrO2Cl2 (D) Cr2O3
Solution: (C). Chromyl chloride test.
Illustration 6:
Brown ring test is used to detect
(A) iodide (B) nitrate (C) iron (D) bromide
Solution: (B). NO3– is detected by brown ring test.
Exercise 3
1) A gas is obtained by addition of dilute H2SO4 to a mixture which turns lead acetate paper black. It is
(A) SO2
(B) CO2
(C) H2S
(D) NO2
2) A substance on treatment with dil. H2SO4 liberates a colourless gas which produces (i) turbidity with baryta water and (ii) turns acidified dichromate solution green. The reaction indicates the presence of
(A) CO32– (B) S2– (C) SO32– (D) NO2–
Illustration 7: Which of the following is not used as a preliminary test for detecting ions?
(A) Dilute H2SO4 test
(B) Charcoal cavity test
(C) Chromyl chloride test
(D) Flame test
Solution: (C). Chromyl chloride test is a confirmatory test for Cl– ion.
IDENTIFICATION OF BASIC RADICALS
Group I
Radicals: Ag+, Pb2+, Hg22+
- Group reagent: Dilute HCl
The radicals are precipitated in the form of their chlorides.
AgNO3 + HCl → AgCl + HNO3 (White ppt.)
Pb(NO3)2 + 2HCl → PbCl2 + 2HNO3 (White ppt.)
Hg2(NO3)2 + 2HCl → Hg2Cl2 + 2HNO3 (White ppt.)
White ppt. of PbCl2 is insoluble in cold water but dissolves in hot water. AgCl and Hg2Cl2 remain insoluble in hot water.
-
Test for Ag+ ions
The white ppt. of AgCl is soluble in NH4OH by forming a complex, while AgCl is again precipitated from the above solution by adding dil. HNO3.
AgCl + 2NH4OH → [Ag(NH3)2]Cl + 2H2O (Diammine silver(I) chloride)
[Ag(NH3)2]Cl + 2HNO3 → AgCl + 2NH4NO3 (White ppt.)
- Test for Pb2+ ions
To the hot solution of PbCl2, add K2CrO4 solution. A yellow precipitate of PbCrO4 is formed which is insoluble in AcOH but soluble in NaOH.
Pb2+ + K2CrO4 → PbCrO4 + 2K+ (Yellow ppt.)
PbCl2 (aq) + 2KI(aq) → 2KCl + PbI2 (Yellow ppt.)
PbI2 + 2KI(excess) → K2[PbI4] (Complex - soluble)
- Test for Hg22+ ions
The white ppt. of Hg2Cl2 turns black on treating with NH4OH.
Hg2Cl2 + 2NH4OH → Hg + Hg(NH2)Cl + NH4Cl + 2H2O (Black)
The black ppt. is dissolved in aqua regia:
3HCl + HNO3 → NOCl + 2H2O + 2[Cl]
2Hg(NH2)Cl + 6[Cl] → 2HgCl2 + 4HCl + N2
Hg + 2[Cl] → HgCl2
SnCl2 + 2HgCl2 → SnCl4 + Hg2Cl2 (White ppt.)
SnCl2 + Hg2Cl2 → SnCl4 + 2Hg (Black)
Group II
- Group II A: Hg2+, Pb2+, Bi3+, Cu2+, Cd2+ (Insoluble in yellow ammonium sulphide)
- Group II B: As3+, Sb3+, Sn2+, Sn4+ (Soluble in yellow ammonium sulphide)
- Group reagent: H2S in acidic medium
The radicals of group II are precipitated as their sulphides.
| Sulphide | Colour |
|---|---|
| HgS | Black |
| PbS | Black |
| Bi2S3 | Brown black |
| CuS | Black |
| CdS | Yellow |
| As2S3 | Yellow |
| Sb2S3 | Orange |
| SnS2 | Yellow |
| SnS | Brown |
Group II B sulphides dissolve in ordinary ammonium sulphide with the exception of SnS. Except HgS, all the sulphides of group II A become soluble in 50% HNO3. HgS dissolves in aqua regia. Copper and Cadmium are separated with the help of KCN.
-
Test for Hg2+ ions
HgS is dissolved in aqua regia and the solution is tested by SnCl2.
3HgS + 2HNO3 + 6HCl → 3HgCl2 + 3S + 2NO + 4H2O
2HgCl2 + SnCl2 → Hg2Cl2 + SnCl4 (White ppt.)
Hg2Cl2 + SnCl2 → 2Hg + SnCl4 (Grey)
-
Test for As3+ ions
The yellow ppt. of As2S3 is insoluble in conc. HCl but dissolves in conc. HNO3. To this solution ammonium molybdate is added, a yellow precipitate is formed.
As2S3 + 10HNO3 → 2H3AsO4 + 10NO + 2H2O + S (boil) (Yellow ppt. - Arsenic acid)
H3AsO4 + 12(NH4)2MoO4 + 21HNO3 → (NH4)3AsO4·12MoO3 + 21NH4NO3 + 12H2O (Yellow ppt. - Ammonium arsenomolybdate)
-
Test for Sb3+ ions
The orange ppt. of Sb2S3 is soluble in conc. HCl. This solution on dilution with water forms a white turbidity.
Sb2S3 + 6HCl → 2SbCl3 + 3H2S (Orange ppt.)
SbCl3 + H2O → 2SbOCl + 2HCl (White turbidity - Antimony oxychloride)
-
Test for Sn2+ ions
The brownish ppt. of SnS is soluble in conc. HCl to give SnCl2. It is tested by HgCl2.
SnS + 2HCl → SnCl2 + H2S
SnCl2 + 2HgCl2 → SnCl4 + Hg2Cl2 (White ppt.)
SnCl2 + Hg2Cl2 → SnCl4 + 2Hg (Grey ppt.)
-
Test for Pb2+ ions
The black ppt. of PbS is dissolved in 50% HNO3. The resulting solution gives a white ppt. on treating with dil. H2SO4.
3PbS + HNO3 → 3Pb(NO3)2 + NO + S + 4H2O (Solution)
Pb(NO3)2 + H2SO4 → PbSO4 + 2HNO3 (White ppt.)
-
Test for Bi3+ ions
The black ppt. of Bi2S3 is dissolved in 50% HNO3. The resulting solution gives white ppt. with NH4OH.
Bi(NO3)3 + 3NH4OH → Bi(OH)3 + 3NH4NO3 (White ppt.)
Bi(OH)3 + 3HCl → BiCl3 + 3H2O
BiCl3 + H2O → BiOCl + 2HCl (Bismuth oxychloride - White ppt.)
2BiCl3 + 3Na2SnO2 + 6NaOH → 3Na2SnO3 + 2Bi + 6NaCl + 3H2O (Black - Sodium stannate/Sodium stannite)
-
Test for Cu2+ ions
The black ppt. of CuS is dissolved in 50% HNO3. To the solution so formed, add K4[Fe(CN)6], forms chocolate ppt.
3CuS + 8HNO3 → 3Cu(NO3)2 + 4H2O + 3S + 2NO
Cu(NO3)2 + K4[Fe(CN)6] → 4KNO3 + Cu2[Fe(CN)6] (Chocolate ppt. - Copper hexacyanoferrate(II))
Cu(NO3)2 + 2NH3 → [Cu(NH3)4]2+ + 2HNO3 (Blue colouration)
-
Test for Cd2+ ions
The yellow ppt. of CdS is dissolved in 50% HNO3. To the resulting solution NH4OH is added slowly, a white ppt. is formed which dissolves in excess of NH4OH.
3CdS + 8HNO3 → 3Cd(NO3)2 + 4H2O + 3NO + 3S
Cd(NO3)2 + 2NH4OH → 2NH4NO3 + Cd(OH)2 (White ppt.)
Cd(OH)2 + 2NH4OH → 2NH4NO3 + [Cd(NH3)4](NO3)2 + 4H2O (Soluble)
[Cd(NH3)4](NO3)2 + H2S → CdS + 2NH4NO3 + 2NH3 (Yellow ppt.)
Group III
- Radicals: Cr3+, Fe3+, Al3+
- Group reagent: NH4OH in the presence of NH4Cl
The radicals are precipitated as their respective hydroxides.
| Hydroxide | Colour |
|---|---|
| Al(OH)3 | Gelatinous white |
| Fe(OH)3 | Reddish brown |
| Cr(OH)3 | Green |
-
Test for Al3+ ions
The white gelatinous ppt. of Al(OH)3 is soluble in NaOH due to formation of sodium metaaluminate. Fe(OH)3 and Cr(OH)3 are insoluble in NaOH.
Al(OH)3 + NaOH → NaAlO2 (aq) + 2H2O
Al(OH)3 + 3HCl → AlCl3 + 3H2O (Soluble - blue litmus)
AlCl3 + 3NH4OH → 3NH4Cl + Al(OH)3 (Blue colour is adsorbed and float in colourless solution)
-
Test for Fe3+ ions
The reddish brown precipitate of Fe(OH)3 is dissolved in HCl. The solution gives an intense blue colour with K4[Fe(CN)6] and blood red colour with KCNS.
Fe(OH)3 + 3HCl → FeCl3 + 3H2O
4FeCl3 + 3K4[Fe(CN)6] → Fe4[Fe(CN)6]3 + 12KCl (Ferric ferrocyanide - Blue colour)
FeCl3 + 3KCNS → Fe(CNS)3 + 3KCl (Blood red colouration - Ferric thiocyanate)
-
Test for Cr3+ ions
The green ppt. of Cr(OH)3 is dissolved in NaOH in the presence of H2O2 and the resulting solution is neutralized with CH3COOH then treated with lead acetate.
Cr(OH)3 + 4NaOH + 3H2O2 → 2Na2CrO4 + 8H2O (Yellow)
Na2CrO4 + (CH3COO)2Pb → PbCrO4 + 2CH3COONa (Yellow ppt)
Group IV
- Radicals: Co2+, Ni2+, Mn2+, Zn2+
- Group reagent: H2S in alkaline medium
The radicals are precipitated as their respective sulphides.
| Sulphide | Colour |
|---|---|
| CoS | Black |
| NiS | Black |
| MnS | Flesh colour |
| ZnS | Dirty white |
-
Test for Co2+ ions
The black ppt. of CoS is dissolved in aqua regia.
3CoS + 6HCl + 2HNO3 → 3CoCl2 + 2NO + 3S + 4H2O
Now, add CH3COOH in excess and KNO2, yellow ppt. is formed.
KNO2 + CH3COOH → CH3COOK + HNO2
CoCl2 + 2KNO2 → Co(NO2)2 + 2KCl
Co(NO2)2 + 2HNO2 → Co(NO2)3 + NO + H2O
Co(NO2)3 + 3KNO2 → K3[Co(NO2)6] (Yellow ppt.)
-
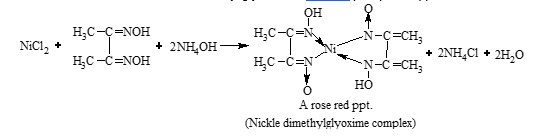
Test for Ni2+ ions
The black ppt. of NiS is dissolved in aqua regia.
3NiS + 6HCl + 2HNO3 → 3NiCl2 + 2NO + 3S + 4H2O
Now, add NH4OH in excess and dimethyl glyoxime, a rosy red precipitate appears.
-
Tests for Mn2+ ions
The flesh coloured ppt. of MnS is dissolved in dil HCl. On adding NaOH to this solution, a ppt. is formed which does not dissolve in excess of NaOH.
MnS + 2HCl → MnCl2 + H2S
MnCl2 + 2NaOH → Mn(OH)2 (Buff ppt. insoluble in NaOH)
-
Tests for Zn2+ ions
The white ppt. of ZnS is dissolved in dil. HCl.
ZnS + 2HCl → ZnCl2 + H2S
The resulting solution is treated with NaOH, white ppt. of Zn(OH)2 appears which dissolved in excess of NaOH.
ZnCl2 + 2NaOH → 2NaCl + Zn(OH)2 (White ppt.)
Zn(OH)2 + 2NaOH → Na2ZnO2 + 2H2O (Sodium zincate - Soluble)
Na2ZnO2 + H2S(g) → 2NaOH + ZnS (White ppt.)
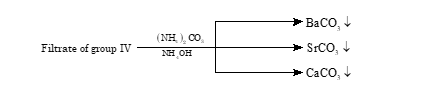
Group V
- Radicals: Ba2+, Sr2+, Ca2+
- Group reagent: (NH4)2CO3 in the presence of NH4OH and NH4Cl
The radicals are precipitated as their respective carbonates (all white ppt.).
-
Tests for Ba2+ ions
The white ppt. of BaCO3 is dissolved in CH3COOH and the solution is treated with potassium chromate, a yellow ppt. is formed.
BaCO3 + 2CH3COOH → (CH3COO)2Ba + H2O + CO2 (White ppt.)
(CH3COO)2Ba + K2CrO4 → 2CH3COOK + BaCrO4 (Yellow ppt. - insoluble in acetic acid)
-
Tests for Sr2+ ions
The white ppt. of SrCO3 is dissolved in acetic acid and the solution is treated with ammonium sulphate, a white ppt. of SrSO4 is formed.
SrCO3 + 2CH3COOH → (CH3COO)2Sr + H2O + CO2
(CH3COO)2Sr + (NH4)2SO4 → 2CH3COONH4 + SrSO4 (White ppt.)
-
Tests for Ca2+ ions
The white ppt. of CaCO3 is dissolved in acetic acid. To this solution ammonium oxalate is added, a white ppt. of calcium oxalate is formed.
CaCO3 + 2CH3COOH → (CH3COO)2Ca + H2O + CO2 (white)
(CH3COO)2Ca + (NH4)2C2O4 → 2CH3COONH4 + CaC2O4 (White ppt. - insoluble in acetic acid)
Group VI
- Radicals: Mg2+, Na+, K+
- Group reagent: There is no common group reagent.
-
Tests for Mg2+ ions
Filtrate of group V + NH4OH + Na2HPO4 → White ppt.
MgCl2 + NH4OH + Na2HPO4 → Mg(NH4)PO4 + 2NaCl + H2O (White ppt.)
-
Tests for K+ ions
To the salt solution add sodium cobaltinitrite, yellow ppt. is formed.
Na3[Co(NO2)6] + 3KCl → 3NaCl + K3[Co(NO2)6] (Potassium cobaltinitrite - Yellow ppt.)
-
Tests for Na+ ions
To the salt solution add potassium pyroantimonate, a ppt. of sodium pyroantimonate is formed.
2Na+ + K2H2Sb2O7 → 2K+ + Na2H2Sb2O7 (Milkiness or ppt.)
Zero Group
Test for NH4+ ions: All ammonium salts on heating with NaOH solution liberate ammonia gas.
(NH4)2SO4 + 2NaOH → Na2SO4 + 2H2O + 2NH3↑
Aqueous solution of ammonium salt when treated with Nessler's reagent gives brown ppt.
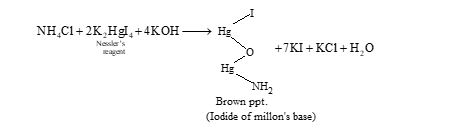
Illustration 8: Three separate samples of aqueous solution of (X) gave following results. One formed white ppt. with excess of ammonia solution, one formed white precipitate with dil. NaCl solution and one formed a black precipitate with H2S. The salt could be
(A) AgNO3
(B) Pb(NO3)2
(C) Hg(NO3)2
(D) MnSO4
Solution: (B)
Pb(NO3)2 + 2NH4OH → Pb(OH)2 + 2NH4NO3 (White ppt.)
Pb(NO3)2 + 2NaCl → PbCl2 + 2NaNO3 (White ppt.)
Pb(NO3)2 + H2S → PbS + 2HNO3 (White ppt.)
- Exercise 4: The brown ring test for nitrate employs
(A) barium chloride (B) ferrous sulphate (C) nitric acid (D) none of the above
- Exercise 5: Which one among the following pair of ions cannot be separated by H2S in dilute HCl?
(A) Bi3+, Sn4+ (B) Al3+, Hg2+ (C) Zn2+, Cu2+ (D) Ni2+, Cu2+
Illustration 9: When a substance (A) reacts with water it produces a combustible gas (B) and a solution of substance (C) in water. When another substance (D) reacts with this solution of (C) it also produces the same gas (B) on warming but (D) can produce gas (B) on reaction with dilute sulphuric acid at room temperature. (A) imparts a deep golden yellow colour to a smokeless flame of Bunsen burner. (A), (B), (C) and (D) are
(A) Na, H2, NaOH, Zn
(B) K, H2, KOH, Al
(C) Ca, H2, Ca(OH)2, Sn
(D) CaC2, C2H2, Ca(OH)2, Fe
Solution: (A)
2Na + 2H2O → 2NaOH + H2↑ (A→C+B)
Zn + 2H2O → Na2ZnO2 + H2↑ (D→B)
Illustration 10: A red solid is insoluble in water. However, it becomes soluble if some KI is added to water. Heating red solid in a test tube produces violet coloured fumes and droplets of metal appear on the cooler parts of test tube. The red solid is
(A) (NH4)2Cr2O7 (B) HgI2 (C) HgO (D) Pb3O4
Solution: (B). HgI2 is scarlet red compound insoluble in water.
HgI2 + 2KI → K2[HgI4] (Soluble)
HgI2 Heat→ Hg + I2 (Droplet - violet vapour)
CHAPTER AT A GLANCE
- Salts of Hg2+, Pb2+, Ba2+ are heavy whereas salts containing carbonate are generally light and fluffy.
- Salts like Zn(NO3)2, MgCl2, ZnCl2 absorb moisture and attain paste like appearance are deliquescent.
- Salts like Pb(NO3)2, Ba(NO3)2 decrepitate, i.e. they produce crackling sound. These salts have mother liquor trapped in crystal structures. During heating when crystal structure crumbles, it produces crackling sound.
- Some salts such as alums, phosphates, borates swell up due to evaporation of water of crystallization.
- During the flame test, the purpose of making paste in conc. HCl is to convert the salt into its respective chlorides which are relatively volatile.
- Bi2(CO3)3 and BaCO3 are not easily decomposed by dil. H2SO4. This is because Bi2(SO4)3 and BaSO4 thus formed are insoluble in water.
- PbCO3 reacts slowly both with dil. HCl as well as dil. H2SO4. This is because PbCl2 and PbSO4 thus formed are insoluble in cold water.
- Chlorides of mercury owing to little ionization do not respond to chromyl chloride test.
- The flame test should be avoided in case of As, Sb, Sn, Pb and Bi salts since they corrode the platinum wire.
- Deliquescent salts: Chlorides of Al, Zn, Cd, Mg or nitrate of Zn absorb moisture from atmosphere to such an extent that finally get dissolved in it and form solution.
- An asbestos fibre can be safely used in place of platinum wire for performing flame test. Glass rod should never be used as it gives a golden yellow persistent colour due to sodium present in it.
- Sulphides of Hg2+, Pb2+, Co2+, Ni2+, Sb2+, Sn2+ are decomposed with dil. H2SO4 only if a pinch of Zn dust is added to the reaction mixture.
Zn + H2SO4 → ZnSO4 + 2[H]
HgS + 2[H] → Hg + H2S
ANSWER TO EXERCISES
Exercise 1: 1) A 2) A 3) B
Exercise 2: A
Exercise 3: 1) C 2) C
Exercise 4: B
Exercise 5: A
Solved Examples
1. When K₂Cr₂O₇ crystals are heated with conc. HCl, the gas evolved is
(A) O₂ (B) Cl₂ (C) CrO₂Cl₂ (D) HCl
Solution: (C). CrO₂Cl₂
2. Microcosmic salt is
(A) Na(NH₄)HPO₄·4H₂O (B) Na(NH₄)·H₂O
(C) Na(NH₃)HPO₄·4H₂O (D) K(NH₄)HPO₄·2H₂O
Solution: (A). Na(NH₄)HPO₄·4H₂O
3. Which among the following is most soluble in water?
(A) Mg(OH)₂ (B) Sr(OH)₂ (C) Ca(OH)₂ (D) Ba(OH)₂
Solution: (B). Among the hydroxides of group II, the solubility increases down the group.
4. Which of the following sulphide has lowest solubility product?
(A) FeS (B) MnS (C) PbS (D) ZnS
Solution: (C).
5. A Chloride is insoluble in cold water but dissolves appreciably in hot water. When placed on platinum wire in Bunsen flame, no distinctive colour is noticed. The cation would be
(A) Mg²⁺ (B) Ba²⁺ (C) Pb²⁺ (D) Ca²⁺
Solution: (C). PbCl₂ is insoluble in cold water but soluble in hot water.
6. CrO₃ dissolves in aqueous NaOH to give
(A) CrO₄²⁻ (B) Cr₂O₇²⁻ (C) Cr(OH)₃ (D) Cr₂(OH)₂
Solution: (A). CrO₃ + 2NaOH → Na₂CrO₄ + H₂O
7. Which of the following is soluble in yellow ammonium sulphide?
(A) CuS (B) CdS (C) SnS (D) PbS
Solution: (C). Among these, SnS is soluble in yellow ammonium sulphide.
[Image: Test tubes showing colored precipitates of different sulphides and a hot wire flame test for chlorides]
8. Which of the following compound gives a red precipitate with AgNO₃?
(A) KI (B) K₂CrO₄ (C) NaBr (D) NaNO₃
Solution: (B). 2AgNO₃ + K₂CrO₄ → Ag₂CrO₄↓ (Red ppt) + 2KNO₃
9. The metal ion which is precipitated when H₂S is passed through the solution which has been acidified with HCl is
(A) Zn²⁺ (B) Ni²⁺ (C) Cd²⁺ (D) Mn²⁺
Solution: (C). Cd²⁺ is in group II A, which is precipitated as CdS in HCl medium.
10. Correct formula of the complex formed in the brown ring test for nitrates is
(A) FeSO₄·NO (B) [Fe(H₂O)₅NO]²⁺ (C) [Fe(H₂O)₄(NO)⁺] (D) [Fe(H₂O)₅NO]³⁺
Solution: (B). Brown ring test for nitrates is due to the formation of [Fe(H₂O)₅NO]²⁺ complex ion.
Assignment Problems
- Which is the hottest part of the flame?
(A) Blue zone
(B) Zone of partial combustion
(C) Zone of complete combustion
(D) Zone of no combustion
- Soda extract is prepared by
(A) fusing soda and mixture, and then extracting with water
(B) dissolving NaHCO₃ and mixture in dilute HCl
(C) boiling Na₂CO₃ and mixture in dilute HCl
(D) boiling Na₂CO₃ and mixture in distilled water
- A precipitate of calcium oxalate will not dissolve in.
(A) acetic acid
(B) HCl
(C) HNO₃
(D) aqua regia
- In the test for iodine, when I₂ is treated with sodium thiosulphate (Na₂S₂O₃) the reaction occurs as, I₂ + 2Na₂S₂O₃ → 2NaI + P. The product ‘P’ is
(A) Na₂S₄O₆
(B) Na₂SO₄
(C) Na₂S
(D) Na₃ISO₄
- When borax is heated on a platinum wire, it forms a glass like bead which is made up of
(A) sodium tetraborate
(B) sodium metaborate
(C) sodium metaborate and boric anhydride
(D) boric anhydride and sodium tetraborate
- Chromyl chloride vapours are dissolved in water and acetic acid and lead acetate solution is added, then
(A) the solution will remain colourless
(B) the solution will become dark green
(C) a yellow solution will be obtained
(D) a yellow precipitate will be obtained
- Which of the following nitrates on strong heating leaves the metal as the residue?
(A) AgNO₃
(B) Pb(NO₃)₂
(C) Cu(NO₃)₂
(D) Al(NO₃)₃
- A mixture on heating with MnO₂ and conc. H₂SO₄ gives brown vapour of
(A) HBr
(B) Br₂
(C) NO
(D) NO₂
- K₂CrO₄ is used to identify
(A) Cu²⁺
(B) Ba²⁺
(C) Ag⁺
(D) Ca²⁺
- On mixing two colourless gases a deep brown gas is formed. The two gases are
(A) N₂O and NO
(B) NO₂ and O₂
(C) NO and O₂
(D) HCl + NH₃
- Which of the following gives blood red colour with KCNS?
(A) Ca²⁺
(B) Fe³⁺
(C) Al³⁺
(D) Zn²⁺
- H₂S on passing through ammonium solution gives white ppt. of
(A) CoS
(B) NiS
(C) MnS
(D) ZnS
- The carbonate of which of the following cation is insoluble in water
(A) Na⁺
(B) K⁺
(C) NH₄⁺
(D) Ca²⁺
- AgCl dissolves in ammonia solution and forms
(A) Ag⁺, NH₄⁺ and Cl⁻
(B) Ag(NH₃)⁺ and Cl⁻
(C) Ag₂(NH₃)₂⁺ and Cl⁻
(D) Ag(NH₃)₂⁺ and Cl⁻
- Colour of KMnO₄ is decolourized without evolution of any gas. The radical present may be
(A) SO₄²⁻
(B) SO₃²⁻
(C) Sn²⁺
(D) both (B) and (C)
Answers to Assignment Problems
1. C
2. D
3. A
4. A
5. C
6. D
7. A
8. B
9. B
10. C
11. B
12. D
13. D
14. D
15. D