The branch of chemistry which deals with the study of the hydrocarbons and their derivatives is called organic chemistry.
Electronic Displacement in Covalent Bonds
Inductive Effect
The displacement of electron along a carbon chain due to presence of a substituent on the carbon atom is called inductive effect. It is a permanent effect and decreases rapidly as the length of carbon chain increases.
Case – I
δδδ+ C₃ → δδ+ C₂ → δ+ C₁ → δ– Z
(–I effect)
(Z – electron withdrawing group)
Case – II
δδδ– C₃ ← δ– C₂ ← δ– C₁ ← δ+ Z
(+I effect)
(Z – electron donating group)
Applications of inductive effect
- Strength of fatty acids: As the number of alkyl groups attached to COOH group increases, the acidic strength decreases. The order of acidic strength is
- (i) HCOOH > CH3COOH > CH3CH2COOH
- (ii) CCl3COOH > Cl2CHCOOH > ClCH2COOH > CH3COOH
- Basic character of amines: Basic nature of amines on the basis of +I effect of alkyl groups should follow the order, (In gas phase or in non aqueous solvents)
2. Basic character of amines: Basic nature of amines on the basis of +I effect of alkyl groups should follow the order, (In gas phase or in non aqueous solvents)
(CH3)3N > (CH3)2NH > CH3-CH2-NH2> CH3-NH2> NH3
tert-amine sec-amine pri-amine methylamine ammonia
However, the basic character of amines has been found to be different and follows the order: sec-amine > pri-amine > tert-amine > NH3. (in aqueous solution)
Trimethylamine is less basic than dimethylamine due to steric factor, i.e. crowding of three methyl groups makes it difficult for a proton to approach nitrogen in order to form a bond.
Electromeric Effect
Temporary polarization of the substrate molecule at the site of multiple bonds, by shift of an electron pair from one atom to the other under the influence of attacking reagents. It is a temporary effect.
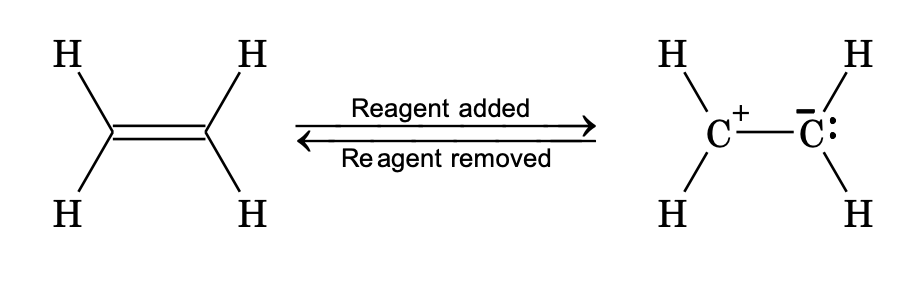
There are two types of electromeric effect.
(i) Positive electromeric effect (+E): Displacement of electron pair towards the attacking reagent.
(ii) Negative electromeric effect (-E): Displacement of electron pair away from the attacking reagent.
Mesomeric or Resonance Effect
The displacement of electron relayed through p electrons of multiple bonds in the carbon chain causing permanent polarization. For resonance effect, it is essential that the atom should have a lone pair of electron or a group containing a p bond. This lone pair of p bond should be in conjugation with the multiple bond of the rest of the molecule.
The conditions required for M or R effect:
1. Molecule should be unsaturated with conjugated system of double bond.
2. Negative charge is in conjugation with double (or multiple) bond.
3. Lone pair of electrons in conjugation with double bond.
The reactivity of compounds is affected by the presence of groups like
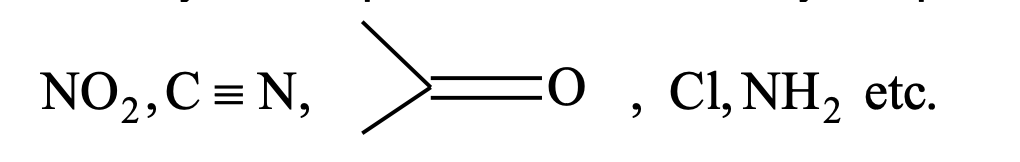
The movement of pai electrons from one end to the other end of the chain through a conjugated system of double bond is observed in resonance effect. It is a permanent effect.
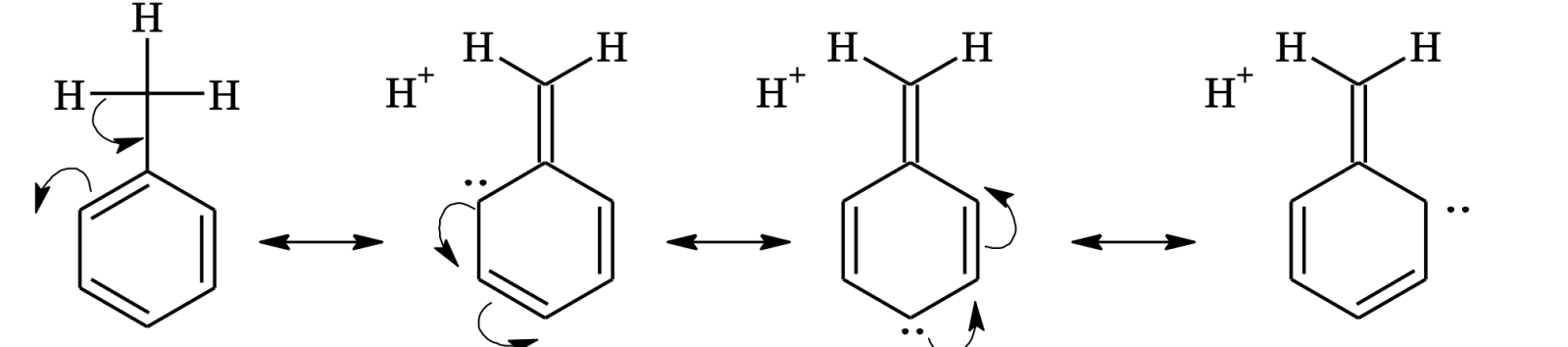
Hyperconjugation (Baker and Nathan Effect)
Shifting of σ electrons induces resonance in rest of the molecule. The delocalization of σ and π bond orbitals is called hyperconjugation.
H H⁺ H H⁺ | | | | H—C—CH=CH₂ ⇌ H—C=CH—CH₂⁻ ⇌ H—C=CH—CH₂⁻ ⇌ H—C=CH—CH₂⁻ | | | | H H H⁺ H⁺
Applications of Hyperconjugation Effect
In the resonating structures, there is no definite bond between carbon atom and one of the hydrogen atoms, hence hyperconjugation is also known as no–bond resonance.
Applications of hyperconjugation effect.
-
- Stability of alkenes: More number of methyl groups attached to double bonded carbon atom more would be the stability of alkene.
CH2 = CH2 < CH3 – CH = CH2 < (CH3)2 C = CH2
No hyperconjugation structures 3 hyperconjugation structures 6 hyperconjugation structures
- Stability of alkenes: More number of methyl groups attached to double bonded carbon atom more would be the stability of alkene.
- Stability of carbonium ions: More number of hyperconjugation structures of the carbocation more will be its stability.
tert–butyl > 9 hyperconjugation structures > isopropy - 6 hyperconjugative structures > ethyl 3 hyperconjugation structures
- Bond lengths: The bond length in a molecule changes if there is hyperconjugation. In C3H3–C2H = C1H2, the C1–C2 bond length is found to be more than 1.34 Å (normal C = C bond length) while the C2–C3 bond distance is less than 1.54 Å (normal C–C bond length).
- Directive influence of the group: +M effect of methyl group in toluene is due to hyperconjugation.
Due to hyperconjugation, there are nine different structures having negative charge at ortho and para positions. Hence, + M effect of alkyl group attached to benzene ring follows the order: methyl > ethyl > isopropyl > tert-butyl.
In the same way, the meta directing influence and deactivating effect of –CCl3 group in benzotrichloride can be explained on the basis of hyperconjugation as follows,
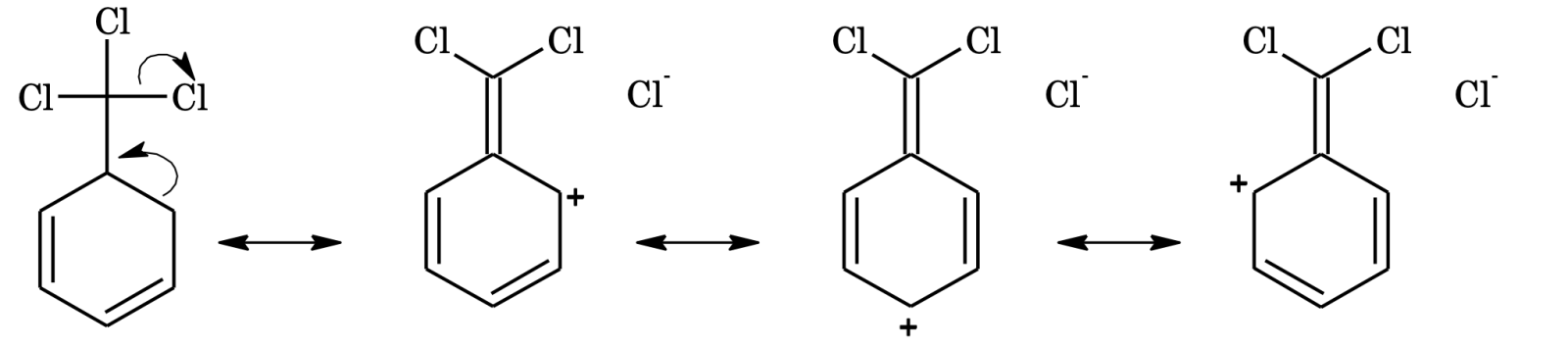
Due to low electron density at ortho and para positions, the meta position becomes point of high electron density, hence electrophilic substitution takes place in meta position.
REACTION INTERMEDIATES
Free Radicals
An atom or group of atoms possessing an odd or unpaired electron, are called free radicals. For example, CH₃˙, CH₃CH₂˙, (CH₃)₂CH˙, CH₂ = CH-CH₂˙, C₆H₅CH₂˙ etc.
The order of stability of free radicals on the basis of resonance inductive effect is as follows:
(C₆H₅)₃C˙ > (C₆H₅)₂CH˙ > C₆H₅˙ > CH₂ = CHCH₂˙ > 3° > 2° > 1° > CH₃˙
Geometry of free radicals: They have trigonal shape and the hybridization is sp². Free radicals are electrophilic in nature.
Example: Which of the following species is most stable?
(A) p–NO₂C₆H₄CH₂˙
(B) C₆H₅CH₂˙
(C) p–ClC₆H₄CH₂˙
(D) p–HOC₆H₄CH₂˙
Solution: (D). It is because of highest +R effect of –OH.
Carbonium Ions or Carbocations
An organic species containing positively charged carbon atom is known as carbocation. The positively charged carbon atom contains six electrons in its valence shell. For example,
CH3+, CH3CH2+, (CH3CH2)2CH+, (CH3)3C+ etc.
More stable carbocation forms at faster rate as compared to less stable carbocation. For example,
(i) R – CH2 – Cl ————> R – CH2+ + Cl−
(ii) R3C – Cl ————> R3C+ + Cl−
The reaction profile of the above two reactions, is given below:
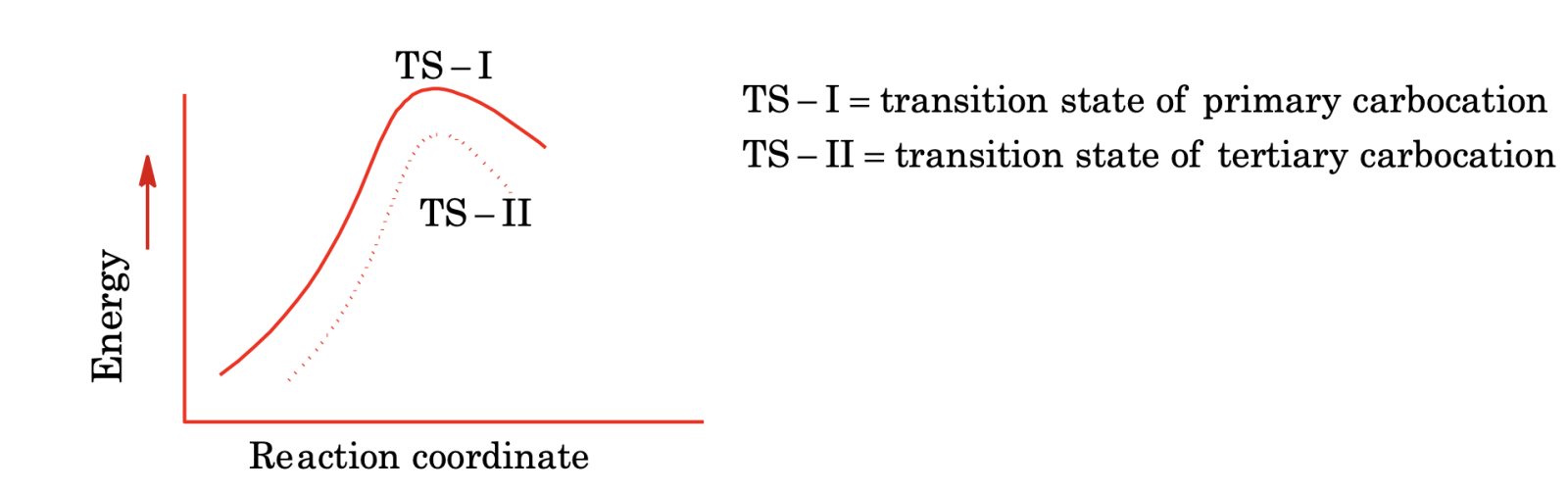
A tertiary carbocation is more stable, the transition state (TS – II) is lower in energy than transition state (TS – I) of primary carbocation. As a result, a tertiary carbocation will form more rapidly than primary carbocation.
Stability of carbocation. The stability of carbocation is similar to free radicals and it is based on resonance hyperconjugation and inductive effect. The order of stability is,
(C6H5)3C+ > (C6H5)2CH+ > C6H5CH2+ > CH2 = CH – CH2+ > 3° > 2° > 1° > CH3+
[Note: Electron attractors (–I effect) increases the positive charge on carbon atom and thus reduces the stability of carbocation, e.g. O2N – CH2 – CH2+, Cl – CH2 – CH2+.]
Geometry of carbocation. Generally, alkyl carbanions are pyramidal in shape but in some cases it may be planar or linear.
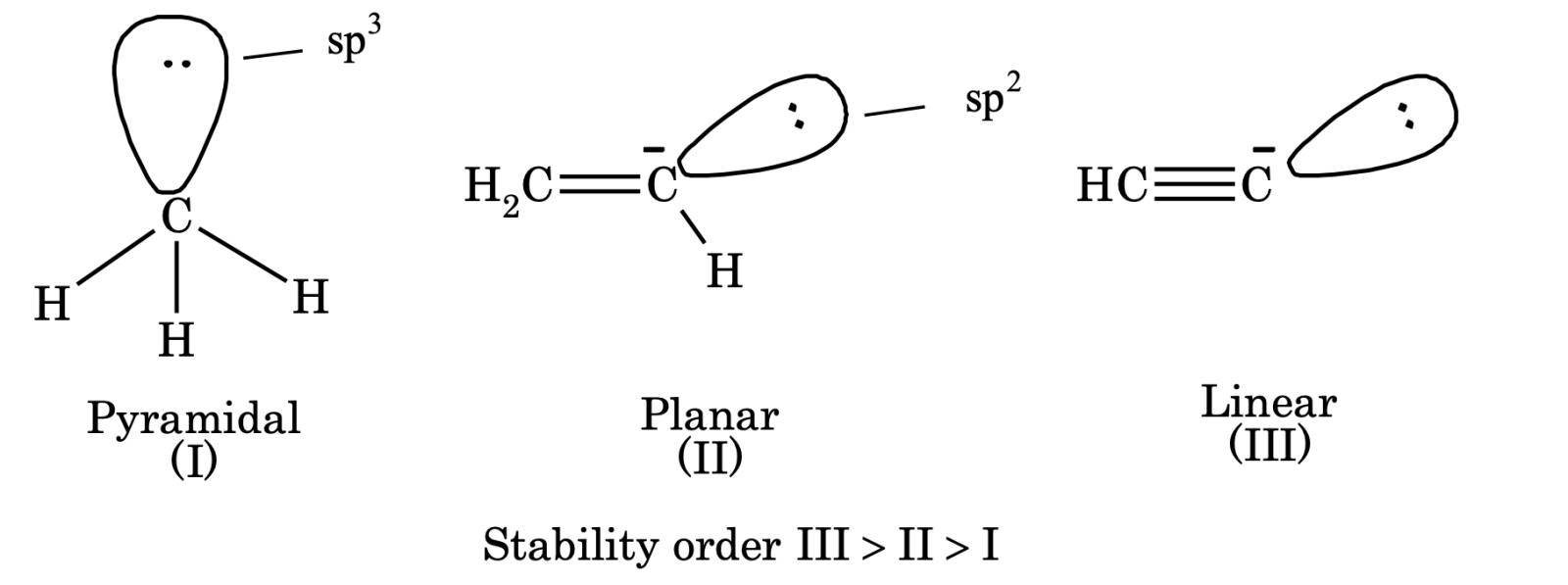
Carbanions are nucleophilic in nature.
Carbene (Biradical)
A short lived divalent carbon atom with two unpaired electrons. It is electron deficient and contains six electrons in valence shell. It is a powerful electrophile due to its reactive nature. For example, :CH2 (methylene), :CCl2 (dichloro carbene) etc.
Carbenes exist in two possible forms
(i) Singlet
(ii) Triplet

Reagents
Reagents are broadly classified into two main categories, i.e.
(i) Electrophiles
(ii) Nucleophiles
Electrophiles
Electrophiles are electron loving species having affinity for electron rich centres.
They are of the following types,
(i) Positively charged: The species having a positive charge, e.g.
H+, CH3+, NO2+, H3O+, NO+, Br+, CH3CO+ etc.
(ii) Neutral: The molecules containing electron deficient atom (i.e. Lewis acids) e.g. :CH2, AlCl3, BF3, ZnCl2, FeCl3 etc.
(ii) Ambident: The molecules with two electron deficient centres, e.g. α, β unsaturated carbonyl compounds.
Nucleophiles
Nucleophiles are electron rich species having an affinity for electron deficient centres.
They are of following types
(i) Negatively charged: The species having a negative charge, e.g. Cl−, Br−, OH−, CN−, NO2− etc.
(ii) Neutral: The molecules having an unshared pair of electrons (i.e. Lewis base), e.g.
:NH3, R − :NH2, R2 − :NH, H2O:, R − :O:, R − O − H etc.
(iii) Ambident: The molecule with two electron rich centres, e.g.
−C≡:N, N=:O, O<−:N−:O etc.
TYPES OF ORGANIC REACTIONS
The organic reactions are classified into four categories.
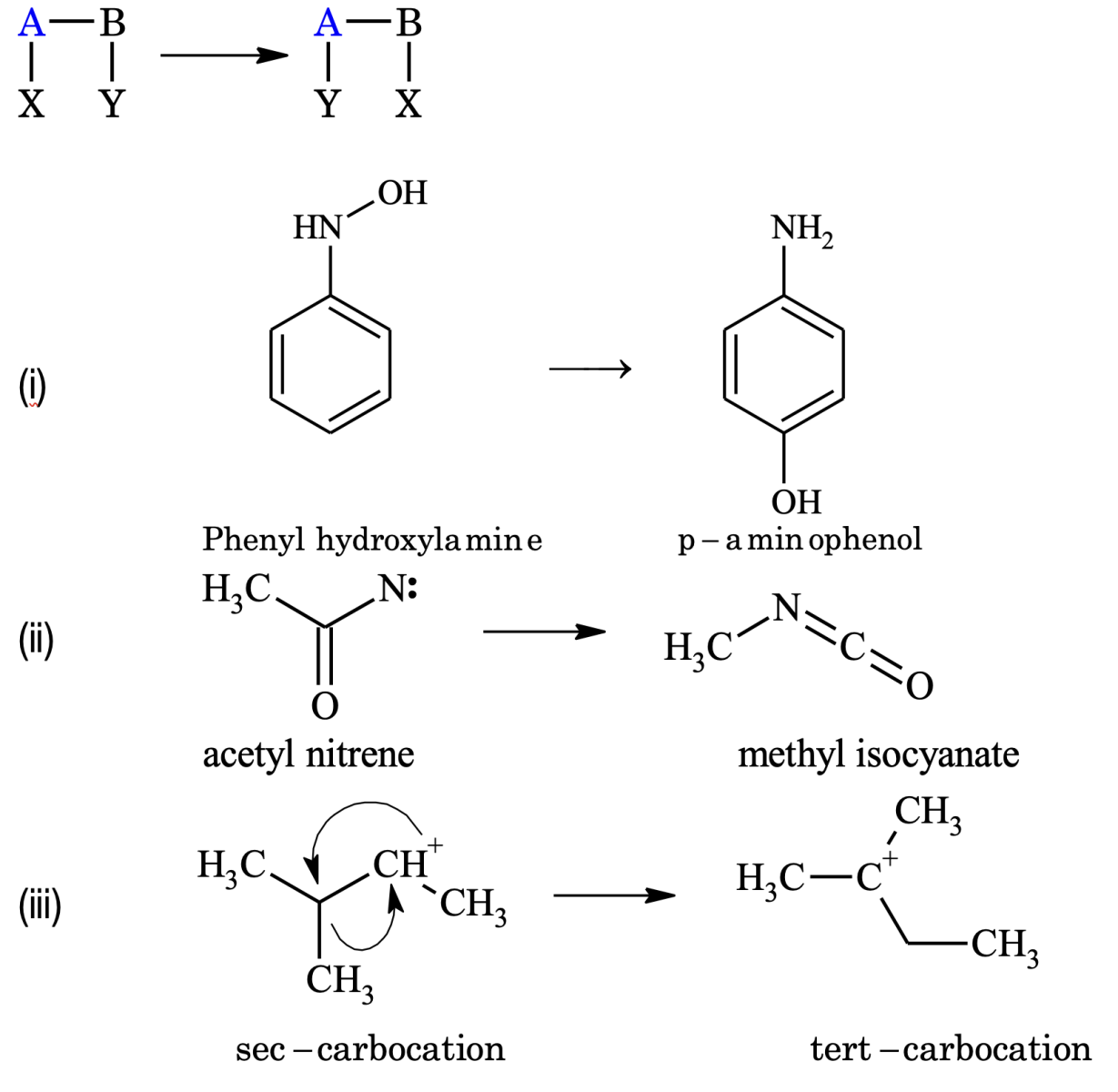
1. Substitution Reaction
The replacement of an atom or group from the organic molecule with another atom or group is known as substitution reaction.
A − B + X − Y → A − X + B − Y
There are three types substitution reaction,
i) Unimolecular substitution reaction (SN1). Tertiary halides undergo substitution reaction by SN1 mechanism.
(CH3)3CCl + OH− → (CH3)3COH + Cl−
Mechanism:
Step – 1 (CH₃)₃C–Cl →slow (CH₃)₃C⁺ + Cl⁻
(intermediate)
Step – 2 (CH₃)₃C⁺ + OH⁻ →fast (CH₃)₃C–OH
(tert–butyl alcohol)
Rate reaction = k[tert–butyl chloride]
Order = 1, molecularity = 2
ii) Biomolecular substitution reaction (SN2). Primary halides undergo substitution reaction by SN2 mechanism.
Chemical Reaction Mechanisms
Mechanism:
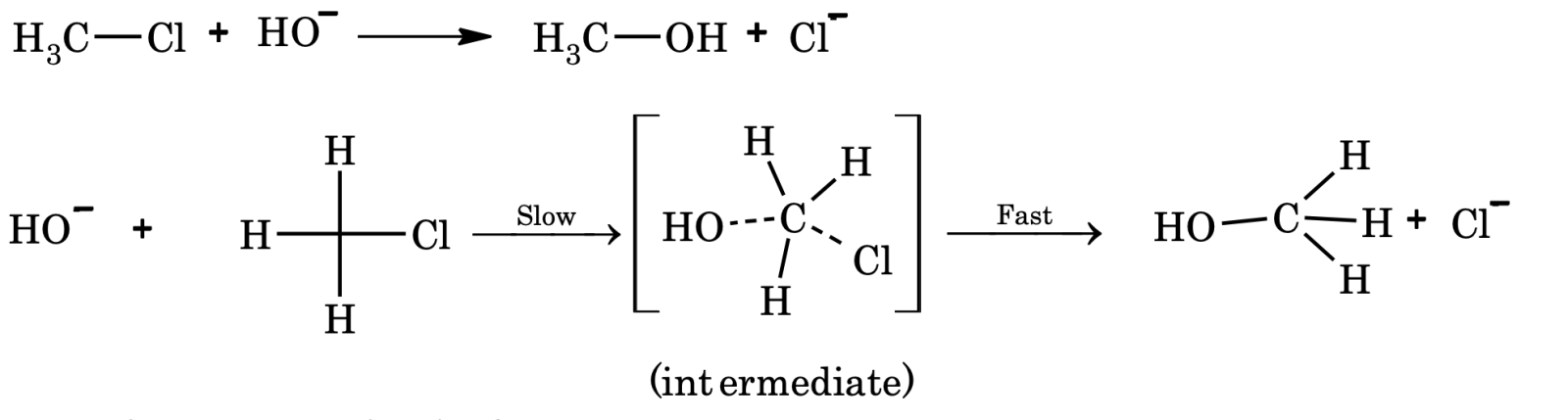
Rate of reaction = k[CH₃Cl][OH&supmin;]
Order = 2, molecularity = 2 iii) Free radical substitution.
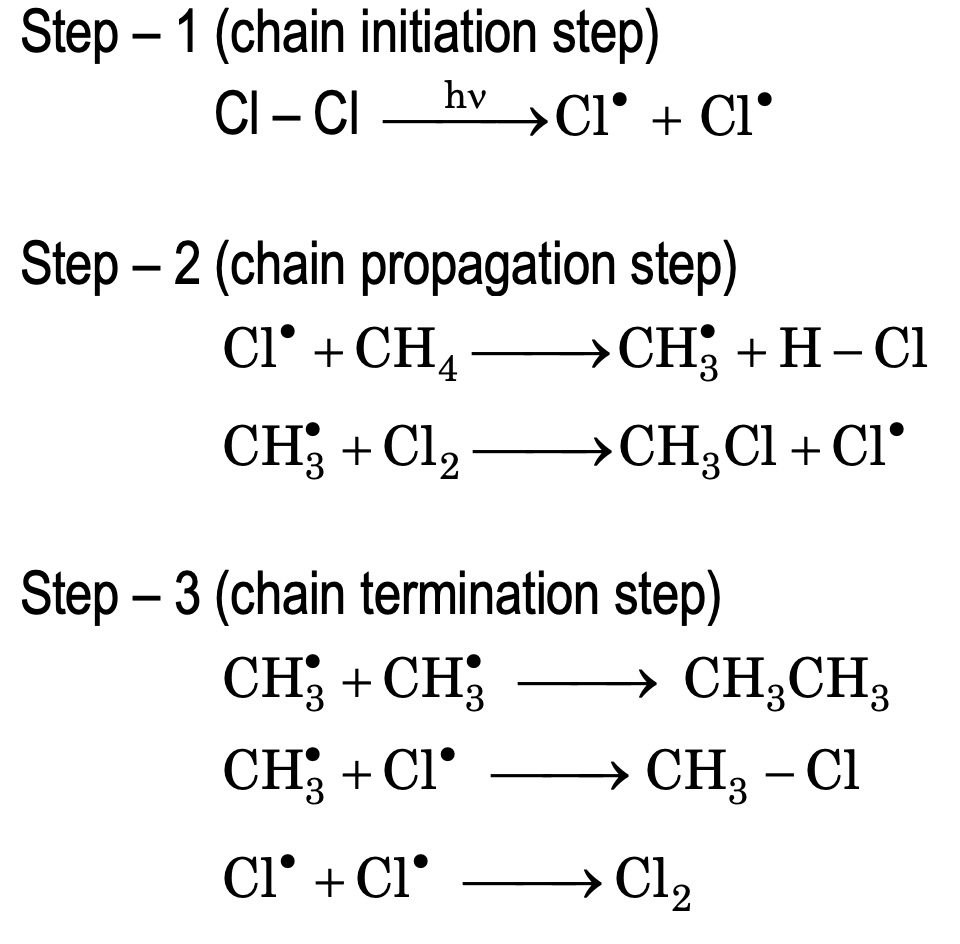
These reactions involve homolytic bond fission and the mechanism involves three steps. Chlorination of methane is the example of free radical substitution.
Mechanism:
Addition Reaction
Usually unsaturated molecules undergo addition reaction. If one π bond is broken then two σ bonds are formed.
A = B + X–Y ⟶ X–A–B–Y
There are three types of addition reaction.
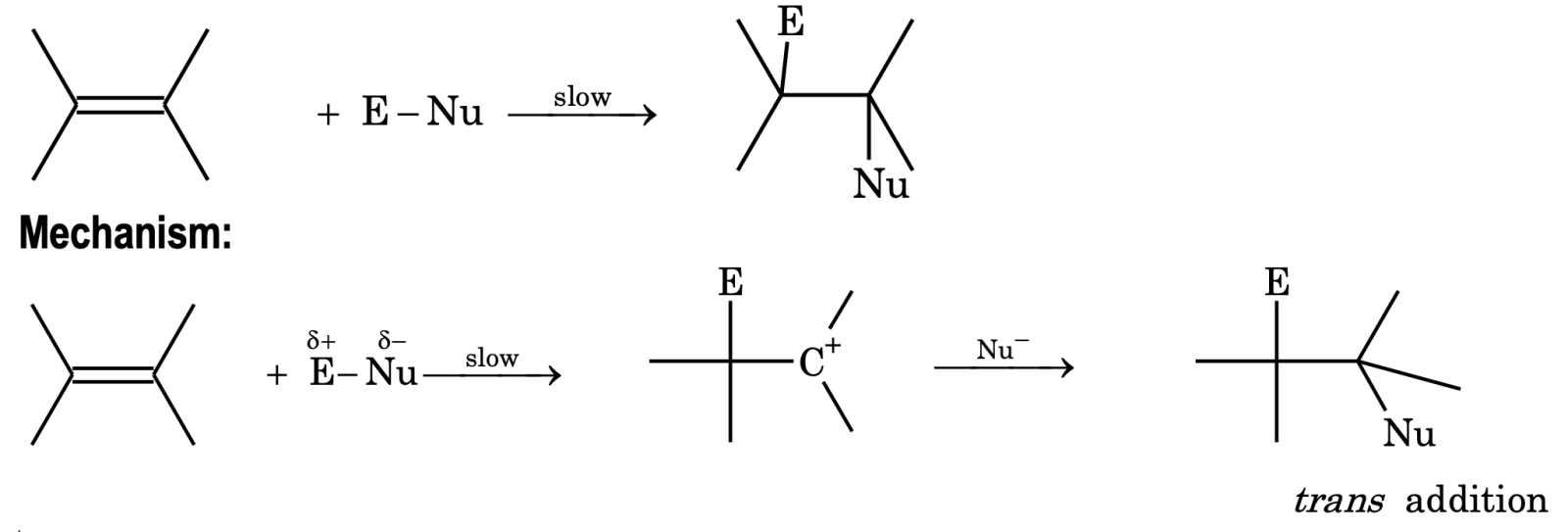
Electrophilic addition. Generally, unsaturated compounds give electrophilic addition reaction.
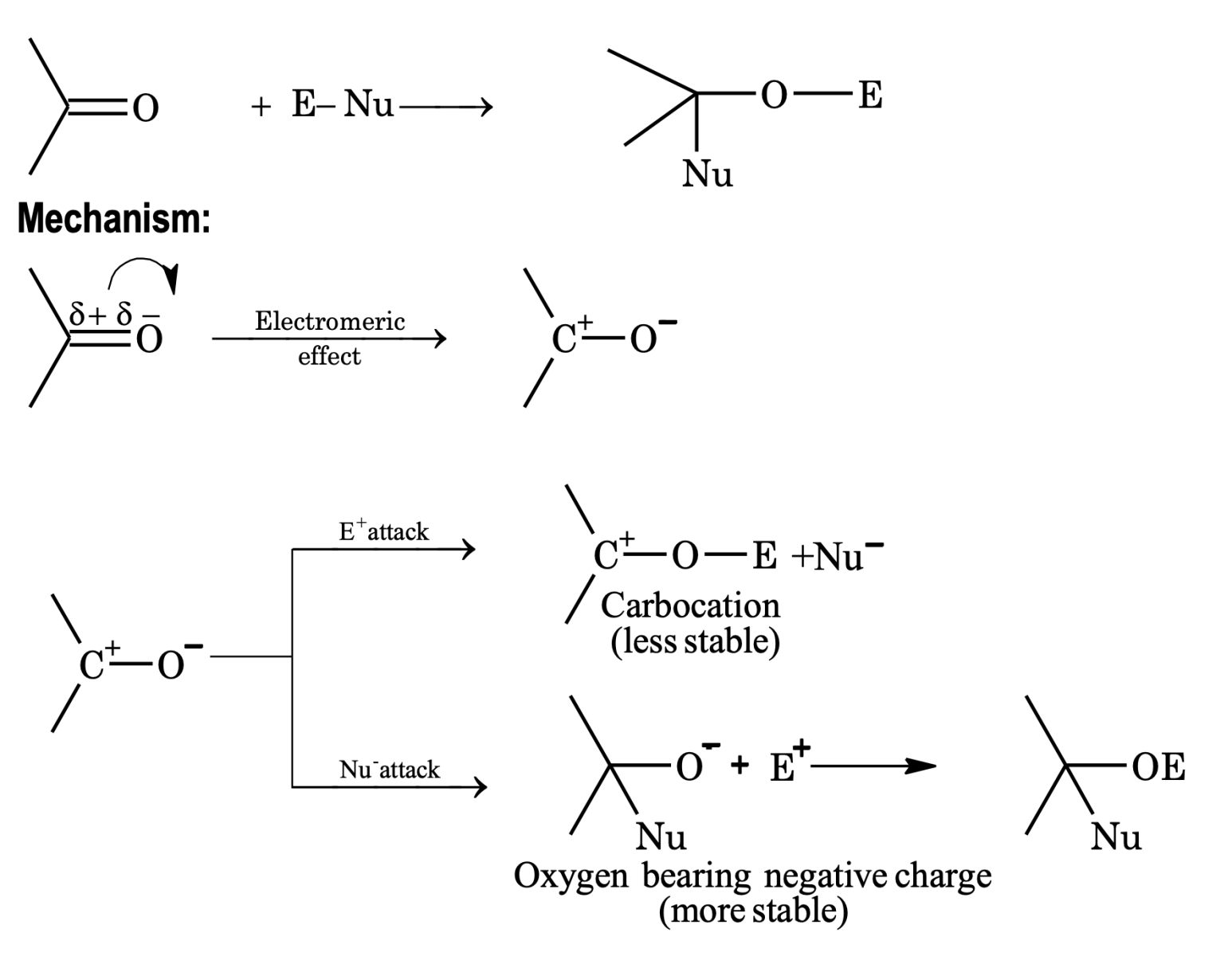
Nucleophilic addition reaction. Generally, aldehydes and ketones give nucleophilic addition reaction.
Free radical addition reaction. Addition of HBr to propene in presence of peroxide gives n-propyl bromide. This is anti-Markonikov or Kharasch effect.
CH₃CH = CH₂ + HBr Peroxide → CH₃CH₂CH₂Br
Elimination Reaction
The loss of atoms or group from adjacent carbon atoms (one in the form of a nucleophile and other in the form of an electrophile) resulting in the formation of an unsaturated compound is known as elimination reaction.
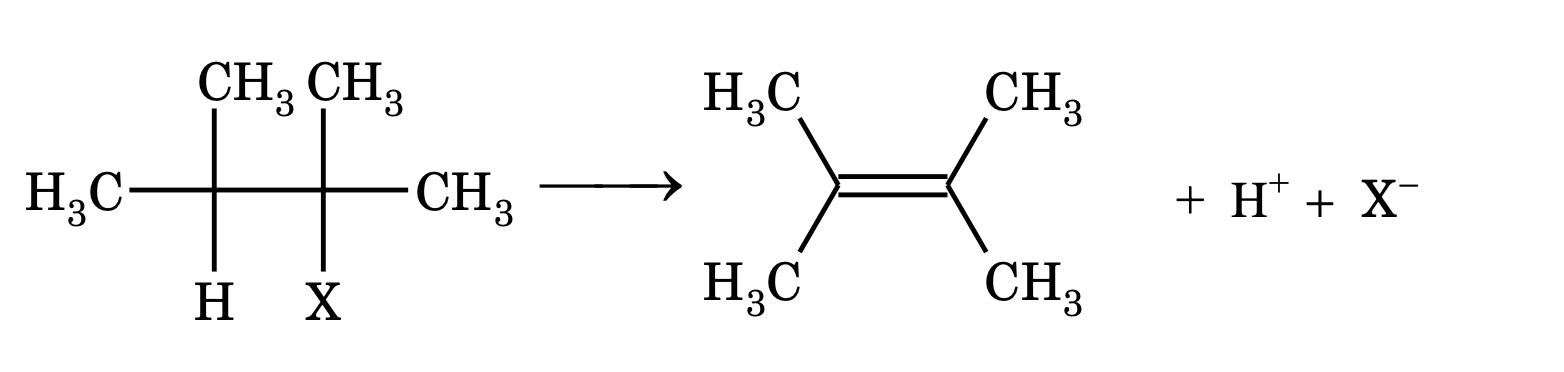
[Note: If these two groups or atoms are removed from adjacent carbon atoms, then it is known as elimination reaction.]
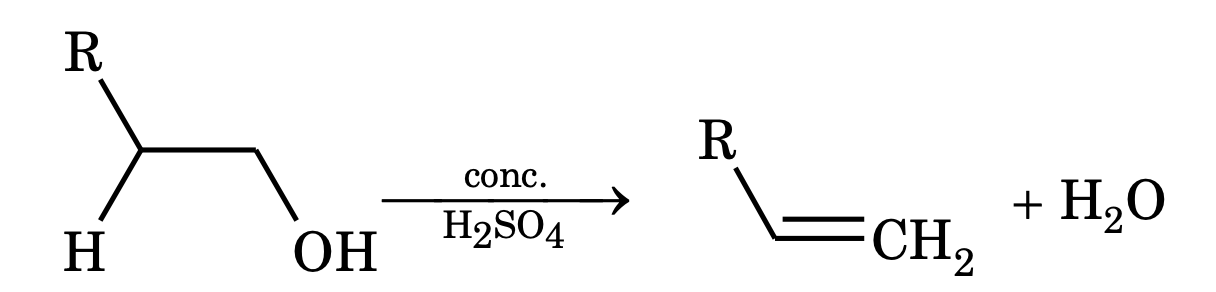
Unimolecular elimination (E1). This reaction involves two steps.
Mechanism:
Step – 1 Heterolysis of substrate gives carbocation and halide ion.
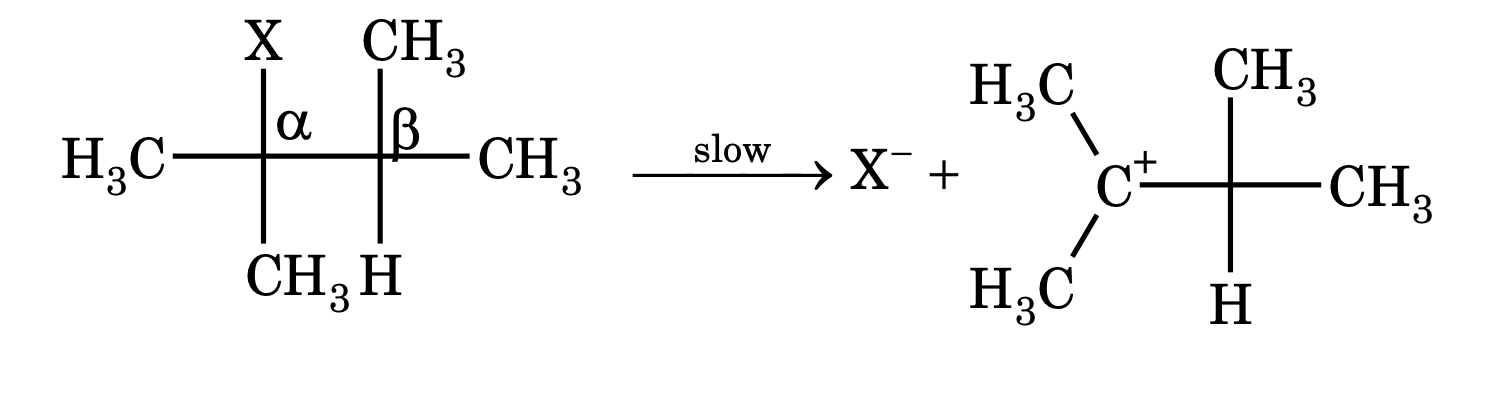
Step – 2: Carbocation gives up proton to a base immediately and alkene is formed.
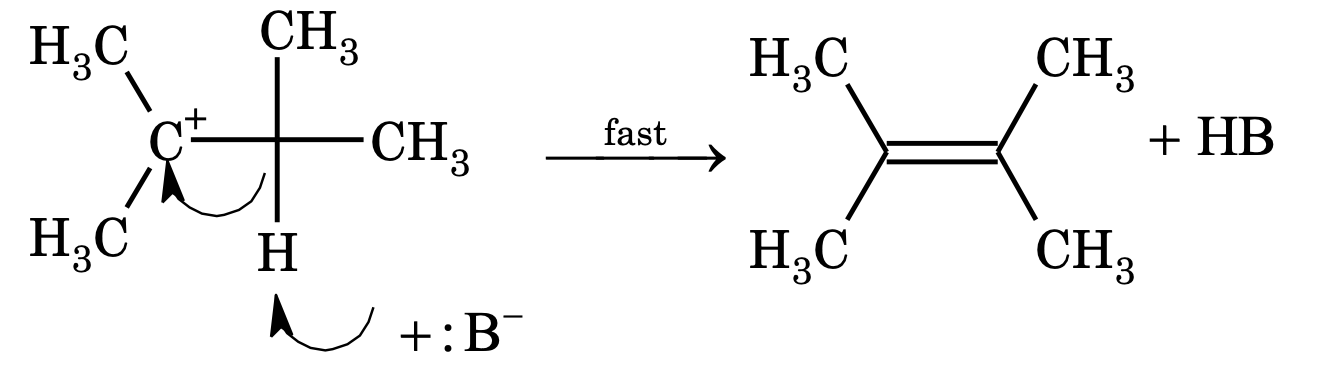
Rate of reaction = k [substrate] [base]
Order =2, molecularity = 2
Rearrangement Reaction
In these reactions, the substituents change their positions.